Date: November, 2018 | Category: Company | Author: Ryan Szporer
The just-released GlobalVision Desktop 5.3 and GlobalVision Web 3.3 each bring exciting, new features to the forefront. The end result, for both deployment options, is greater overall ease of use, though.
Top new GlobalVision Desktop features include:
- Text Inspection tweaks to insertions/ deletions, which are now easier to locate, along with the new ability to search for text when creating zones and resynching differences.
- New user-friendly keyboard shortcuts are listed in a readily accessible in-app guide.
Top new GlobalVision Web features include:
- A face-lifted Graphics Inspection Mode, complete with adjustable Flash, Master, and Sample panels.
- Mozilla Firefox support.
Improved Insertion and Deletion Detection
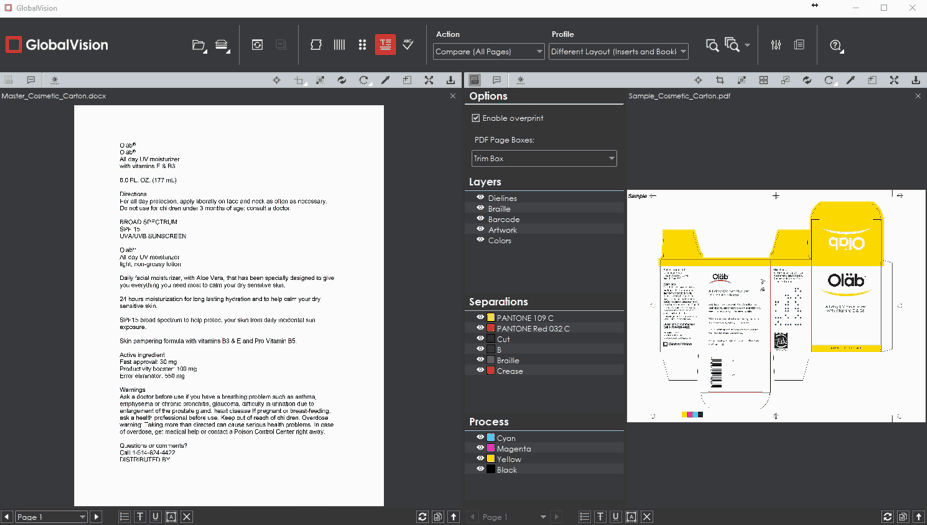
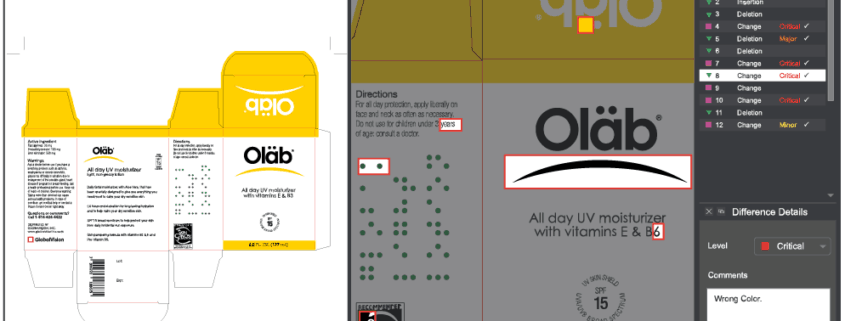
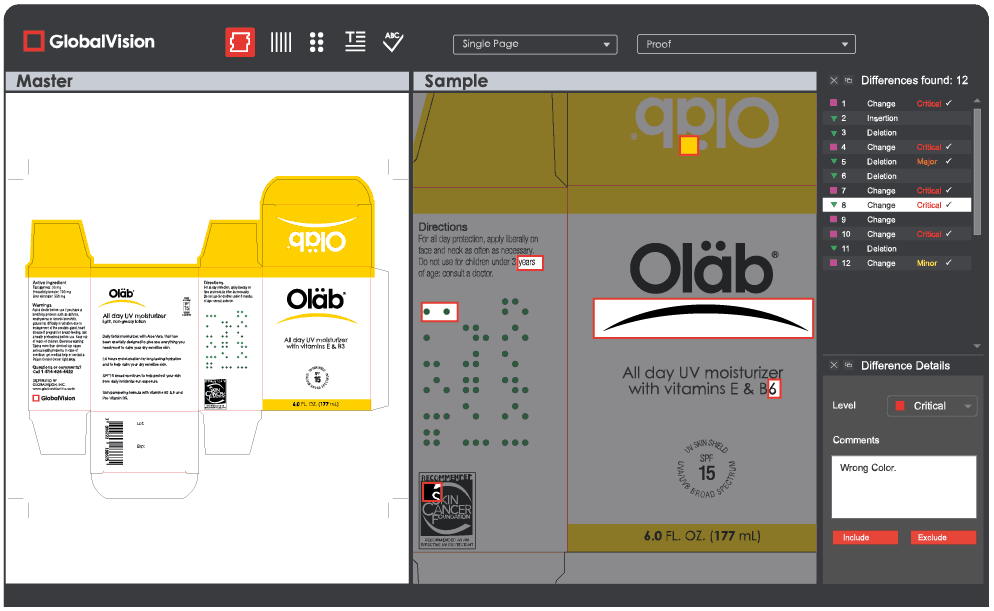
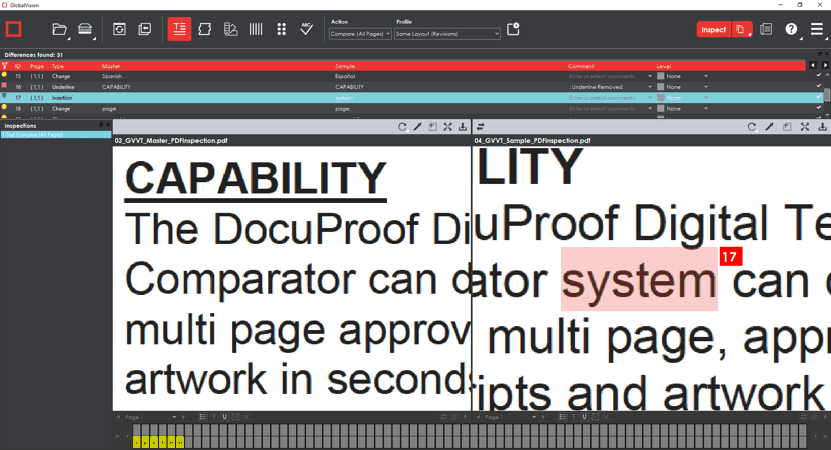
GlobalVision Desktop users can now make use of a new search function. As the text is being selected to create a “zone,” possible matches in the opposite panel now pop up. Much the same mechanic lets users match text when resynching differences after the inspection has been run.
Also following inspections: Detected insertions and deletions get highlighted in red in only the corresponding panel, letting users easily determine the difference’s exact location.
The Wait for the (Bigger) Flash Is Over
GlobalVision Web users running graphics inspections can now make the Flash panel as big as they want. The panel, which flashes between the Master and Sample in rapid succession to highlight differences, can be resized or popped out as a separate browser window. Meanwhile, the Master and Sample panels can be toggled off altogether, allowing for a fully customizable inspection view.
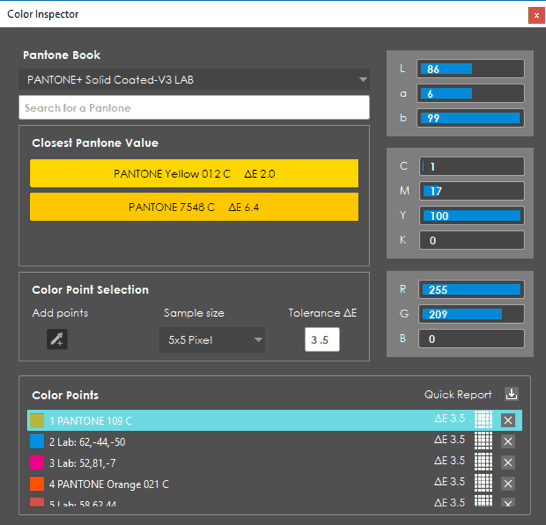
More of Color Inspection Mode
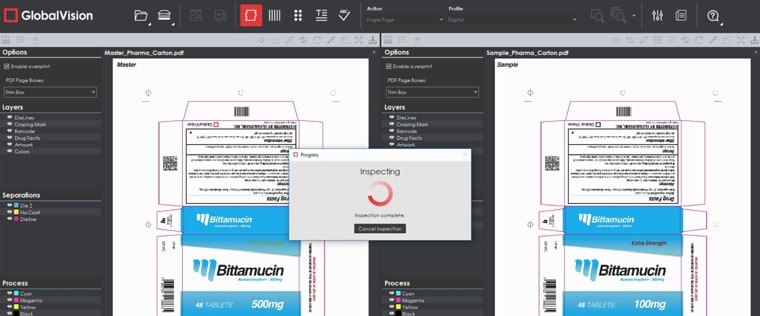
Color Inspection Mode, which innovatively confirms the accuracy of Pantone, LAB, CMYK, and RGB values at select points, has also been updated. A streamlined, new look to the user interface now accompanies an additional inspection “action.” Point to Point inspections can now be run, where, instead of having GlobalVision Desktop automatically compare the same point on the Sample to the one on the Master, users specifically pick points on each file to compare to each other.
Other Updates:
- Sleeker designs coupled with general user-interface improvements.
- U.S. Postal Service 4-State barcode support in GlobalVision Desktop Barcode Inspection.
- Available French, German, and Spanish translations of GlobalVision Desktop itself.
- Simpler processes to regain access to locked-out accounts in both versions.
Before, locked-out GlobalVision Desktop and Web users had to contact administrators to log back in. Now, they can re-activate their accounts on their own through an email notification (Web) or by simply waiting until their system’s pre-determined lock-out period ends to try logging in again (Desktop). The forgotten-password retrieval system has also been revamped in GlobalVision Desktop, which emails a temporary password to users upon request. Hassle-free.
Ultimately, it’s one of many additional features designed to simplify the lives of GlobalVision users. For a complete list, read the release notes for GlobalVision Desktop 5.3 and GlobalVision Web 3.3.
_________________________________________________________________________________________
Ensure your content is always error-free in record time with GlobalVision. Try it now for free.
Keep up with the latest updates in automated proofreading software. Sign up for our newsletter.