New Technologies Every Pharmaceutical Packaging Company Should Consider
Date: September, 2017 | Category: Proofreading | Author: Marvin Magasura
Nowadays, pharmaceutical packaging companies are experiencing big market growth. Reaching an expected compound annual growth rate (CAGR) of CAGR of 11.34% from 2021 to 2028, the market is witnessing particularly quick development in the BRIC countries and throughout Asia. Given this rapid rise, financial experts claim that the market could be worth USD 957.59 billion in 2028.
We live in an era where healthcare systems need to provide for an aging population with increasing incidences of chronic diseases. So, it’s no wonder that the need for pharmaceutical companies (and packaging solutions) is at an all-time high.
On the other hand, the pharmaceutical packaging industry is undergoing a change of its own. The needs of the consumer are constantly evolving, cost pressure is mounting, safety regulations are becoming stricter every year, and manufacturers are facing the challenge of finding new and creative solutions to several pressing issues.
As a result, evolution is paramount in order to achieve the ultimate goal: providing high-quality services for patients while also keeping costs down.
Every Pharma Packaging Company’s Dream: Complete Serialization
New serialization technologies are being introduced in the market every year proving that, even today, serialization is still one of the biggest driving forces behind the changing pharmaceutical packaging industry.
In the past years, Japanese pharma packaging companies have widely adopted one of the most exciting new technologies so far: 2D barcode printing directly into vials, pills, and capsules. It seems like this trend has been catching on in the Americas recently, as well.
By bringing serialization directly onto pills, a new issue arises. Is the technology ready to read and interpret smaller print? While barcode reading technology is not very well developed, we are likely to witness, in the coming years, new devices to interpret and track these microscopic serialized codes.
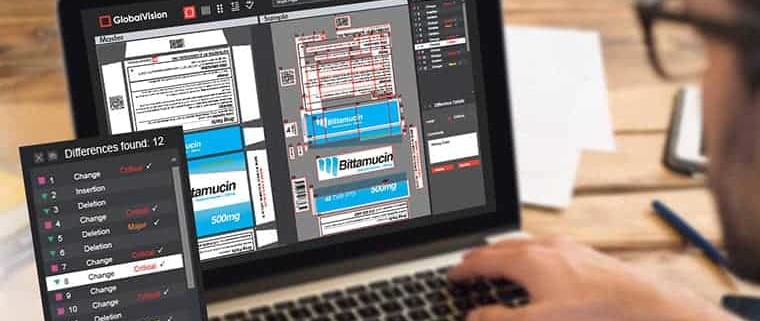
GlobalVision’s Barcode Inspection tool is one of the few quality control software tools that can automatically locate and grade all barcode types instantly, without having to actually print them – removing the need to verify with a device.

Biological Drugs Forcing Pharma Packaging Changes
Biological drugs, like vaccines, antibodies, and interleukins, are very different from conventional small-molecule formulations because their structure resembles substances made by living organisms. In the last decade, we’ve seen how new cancer treatments are shifting more and more towards bio-drugs, but treatments for other diseases are crossing over too.
This rising popularity continues to fuel unanticipated pharmaceutical packaging changes due to drug interactions with packaging materials, leading to increased demand for new formats and materials.
A pharmaceutical packaging format that is undergoing serious change is the glass vial. Some new biological drugs have been found to delaminate glass, resulting in undesired glass flaking in solutions. Manufacturers around the globe are now searching for a way to change how vials are made in order to mitigate unwanted delamination effects.
Another medical instrument undergoing severe scrutiny is the prefilled syringe. Some new biologics have reacted to components used in the production of syringes, often causing protein denaturation and therefore aggregation of their active substances.
Materials like silicone oil (used for the lubrication of syringe plungers) or tungsten (used in the formation of the glass syringe tip) have been linked to causing these reactions. The industry is now looking for a way of developing new silicone and tungsten-free syringes.
Human vs. Automated Pharmaceutical Packaging
The ever-present question of human vs. automated quality control for pharma packaging is still making content for countless blogs all over the web. However, sufficient time and research have concluded that automation is the biggest and most valuable investment your company could make.
Monotonous and repetitive tasks are not the best jobs for humans, especially when accuracy is extremely important. Human mistakes are without doubt the first cause of labeling issues, resulting in complete recalls. Automatic verification software solutions are needed in order to achieve absolute accuracy.
What you might save on investment, by using the manual method, you’ll lose in the cost of human labor and unintentional mistakes. The risk for errors is even higher with concurrent activities, such as the line operator checking serialization while also moving product containers.
Another big concern is product security. Automated packaging, palletizing, or case-packing protects the drugs from any harm at the location, in logistics, and in repackaging. While the investment may be seen as high, it’s a proven fact that efficiency will increase, and the ROI will be noticeable very quickly.
Automation is not that difficult to acquire, anyway. GlobalVision provides an all-in-one platform for packaging and labeling quality control for supply chains worldwide. Integrating text, graphics, barcode, spelling, braille, and print inspection, GlobalVision is one of the top quality control providers in the pharmaceutical packaging business.

Childproof vs. Senior-Friendly Pharma Packaging
Creating childproof containers is paramount for the pharma packaging industry. Given the powerful effect of today’s drugs, children must not have access to the content of the containers, and this is ever more the case as brand-new guidelines and regulations are being passed every year, meaning more products will need to be packaged in childproof containers.
Undoubtedly, the need for childproof packaging could impact another urgent concern: senior friendliness. There is a growing number of older people suffering from chronic diseases, and they need to be able to access their medication easily. How can pharmaceutical packaging companies deal with the problem of developing containers children can’t open but older people can?
The answer lies within package design. Newly developed opening mechanisms rely less on dexterity and more on knowing how the container opens. The MedLock EZT, for instance, which was created by Colbert Packaging in 2014, uses integrated locking mechanisms. These work by squeezing and pressing touchpoints at one end, while also sliding the blister card through the other.
Another example is Locked4Kids, launched by EcoBliss and labeled as being “difficult for kids, easy for adults“. This mechanism consists of a carton box (designed for packing medication) that has many push points aligned diagonally on both sides and only when pressed together will they open the package. The distance between these push points is too large for kids’ hands to cover, making it physically impossible for them to open the box.
Increased Security to Avoid Counterfeiting
Developing countries are still facing serious counterfeiting issues. So, improving supply-chain security must be a top priority for pharma companies. The natural trend right now is moving towards clever labeling solutions in order to stop package tampering.
Certain pharmaceutical packaging companies are already implementing new ways to make medical products more traceable. For instance, Tracetag’s ValiMark is a substance that can be mixed with many ink systems and guarantees maximum traceability. Other companies are using features like tamper-evident stickers to avoid package interference, holographic foils for authentication, and even radio frequency ID tags to make inventory control simpler.
Either way, added security is still necessary in order to prevent counterfeiting, which is a major cause of morbidity, mortality, and loss of public confidence in drugs and healthcare companies.

Different Standards Require Flexibility
Navigating different standards within markets is a challenge that pharmaceutical packaging companies continue to face, even for next-door markets that only present slight differences. While we may dream of one day achieving a global standard for pharma packaging, that day is still a long way ahead of us. That’s why this current challenge keeps on fueling the need for flexible equipment.
Drug manufacturers that produce for several countries on a single processing line need to change their serialized data according to every market’s standards.
Fortunately, GlobalVision’s Text Inspection lets companies compare two, unlike documents to manage the accuracy of the text, while also checking for compliance with US and EU standards and maintaining data integrity. Another benefit of automated proofreading is that even the smallest error can be discovered, reducing errors that escape the human eye.
Temperature-Stable Pharma Packaging Solutions
These days, drug supply chains are spreading globally. Medical products might need to be transported countless miles across many different climate conditions in order to reach a certain market. Some products might endure it, but plenty will be irreversibly damaged. So, it’s imperative that secondary pharmaceutical packaging is capable of achieving and maintaining temperature stability.
In the old days, we grew accustomed to using dry ice or gel packs, but smart pharma packaging technology is quickly becoming the law of the land. Materials like expanding polystyrene, bio-friendly bio foam, or temperature-stable packaging are gaining momentum, offering a long-term solution.
GlobalVision is the leading developer of quality control technologies for retail and pharmaceutical packaged goods. Learn how GlobalVision has provided new business opportunities across all industries with an extra layer of security.
Request a free trial for GlobalVision Digital Inspection Solution.
Learn More about Pharmaceutical Trade Tips for the Printing Industry
Remain FDA-compliant by ensuring your labels are consistently meeting labeling requirements. Get your complete guide here.








Recent advances in packaging design, materials, and technologies mean that today, there is a wide variety of user-friendly primary and secondary packaging options to choose from.