Down to The Last Period 

A Guide to EU Pharma Regulatory Compliance: Everything You Need to Know
Date: April, 2024 | Category: Compliance | Author: Hana Trokic Understanding the complex regulatory compliance landscape of the European Union’s (EU) pharmaceutical industry poses a significant challenge for companies aiming to…

GlobalVision x Drupa 2024 – We’re Going to the Print Technology Event of the Year
Date: April, 2024 | Category: Company | Author: Hana Trokic GlobalVision is thrilled to announce our participation in Drupa 2024, set to take place in Dusseldorf, Germany from May 28th - June 7th, 2024. The largest and most…
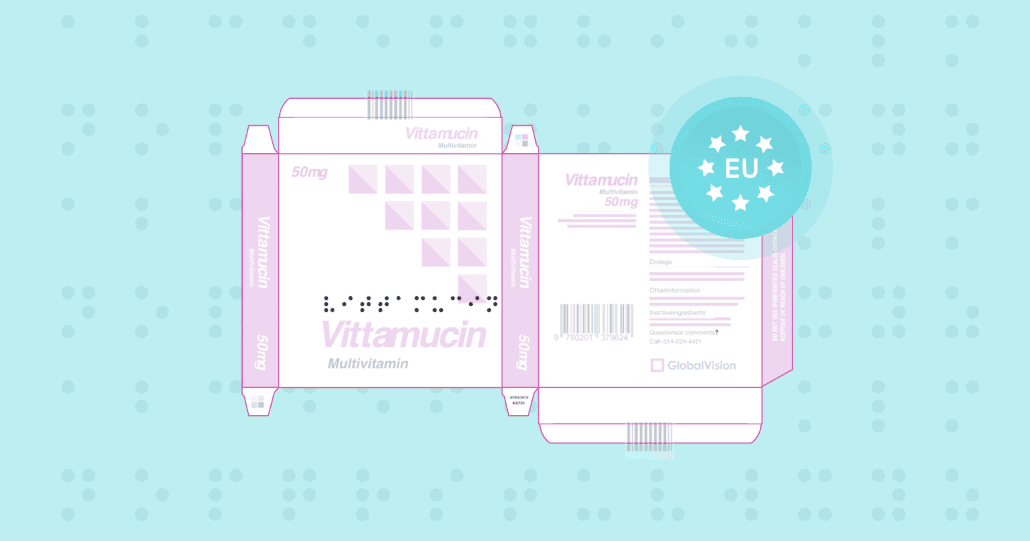
GVD Braille Height Sets A New Standard for Precision and Speed in Braille Inspections for EU Pharmaceutical Market
Date: April, 2024 | Category: Compliance | Author: Hana Trokic GlobalVision’s Braille Height is breaking new ground by offering unmatched precision and speed never before seen for regulatory Braille inspections. With the new GVD…

10 ChatGPT Prompts to Enhance Pharmaceutical Proofreading
Date: April, 2024 | Category: Proofreading | Author: Hana Trokic With AI, pharmaceutical companies can now streamline the process of analyzing vast amounts of data and documentation, ensuring compliance with regulatory standards and…

The Verify x Esko WebCenter Integration Automates Proofreading and Inspections for Packaging Artwork
Date: March, 2024 | Category: Proofreading | Author: Hana Trokic The Verify x Esko WebCenter Integration represents a digital shift in the packaging artwork review process.
Verify x Esko WebCenter - An Integration to Unify Your…








