Compliance Updates and Blog Posts
Keep Reading 

Verify’s New AI-Powered OCR Feature Brings Unmatched Character-for-Character Inspections to Regulated Industries
Date: February, 2024 | Category: Company | Author: Hana Trokic Verify's New AI-Powered OCR Feature is Here! Verify, GlobalVision’s newest and most innovative cloud-based proofreading software, has just raised the bar for proofreading…

Why Regulatory Affairs Teams Need Document Comparison Software in 2024
Date: February, 2024 | Category: Proofreading | Author: Hana Trokic Why do regulatory affairs teams need document comparison software in 2024? For Regulatory Affairs, where adherence to strict compliance standards and efficient…
Medicine Packaging: Navigating Regulations in the UK
Over the years, requirements of medicine packaging have undergone significant changes, driven by advancements in technology, changes in consumer expectations and needs, and, most importantly, the ever-evolving stringent regulations imposed by regulatory agencies such as the MHRA.
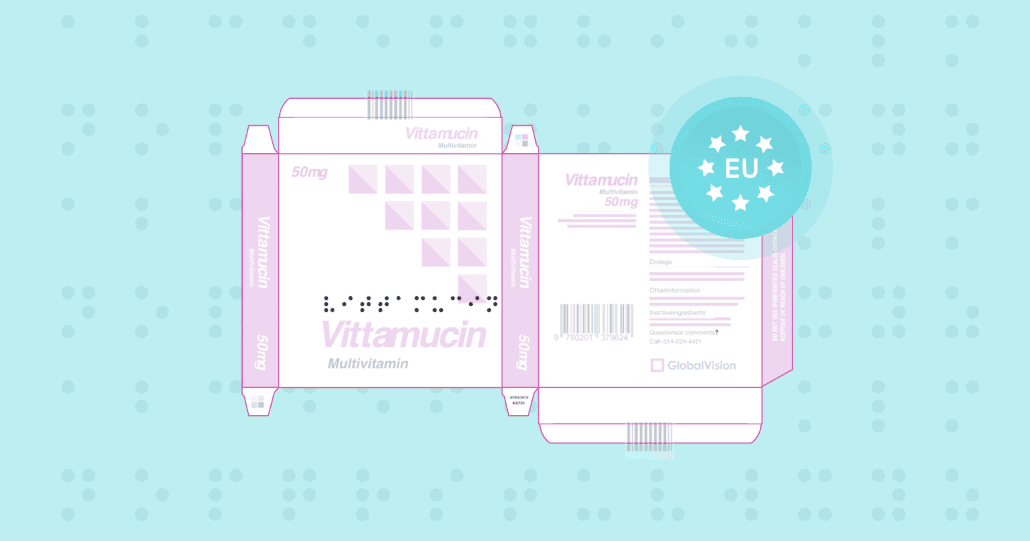
Revolutionizing Braille Inspection: Introducing the Upgraded GVD Braille Module
From a quality control perspective, Braille Inspection and accuracy play an important role as Braille is mandatory on pharmaceutical packaging all across Europe, and is strongly recommended by the FDA in Western markets, while other regions such as the ASEAN markets are working to heighten inclusivity by implementing braille requirements on packaging as well.

Dempsey Corporation Transforms the Label Review Process with GlobalVision
After exploring various solutions on the market, Dempsey Corporation made the decision to incorporate GlobalVision into their quality control processes. to eliminate errors and fatigue caused by manual proofreading.

A Beginner’s Guide to Sustainable Packaging
While “going green” may be a proud point for many businesses in this day and age, in the world of packaging and design, it’s not necessarily going far enough. Instead, it’s all about being sustainable.
Compliance in Medicine Post Brexit: Everything You Need to Know
As we continue to process the historic decision of the United Kingdom to exit the European Union, commonly known as Brexit, its far-reaching implications continue to unfold across various sectors. In terms of healthcare and pharmaceuticals, the repercussions are expected to be particularly noticeable and profound.

A Definitive Guide to Data Integrity Assurance
In the digital age, where data serves as the backbone of decision-making, business operations, and technological advancements, ensuring the integrity of data has become paramount. From sensitive information to critical corporate records and beyond, the accuracy, consistency, and reliability of data play a pivotal role in building trust, driving innovation, and avoiding potentially catastrophic consequences.

Exploring Effective Security Measures: Safeguarding Data and Building Trust
In today’s digital age, information security has become an utmost concern for organizations and their customers. As cyber threats continue to evolve, businesses must prioritize safeguarding sensitive data and ensuring its confidentiality, integrity, and availability.

IPG Health Increases Efficiency in Document Reviews With GlobalVision
IPG Health is a network of world-renowned agencies focused on health communication and marketing. Collectively, the network is made up of over 45 agencies that are spread across six continents and 6,500+ employees driven by a company-wide obsession with harnessing creativity, technology, science, and data to inspire behaviors that fuel better health.

Accelerating Success: GlobalVision Revolutionizes iNova Pharmaceutical’s Product Launches with Unmatched Accuracy and Speed
iNova Pharmaceuticals is a global organization dedicated to the development, marketing, and distribution of a diverse range of prescription medications and consumer health products.

How to Master FDA Labeling Requirements: Your Comprehensive Guide is Here
This comprehensive guide provides detailed insights into FDA labeling requirements and ensures your organization not only thoroughly understands labeling guidelines, but also sets best practices in place to consistently meet FDA labeling requirements with complete ease.
Your Complete Guide to Meeting FDA Labeling Requirements
FDA labeling requirements vary from market, product, and commodity. Each product, depending on its intention of use, has its own set of requirements that manufacturers need to follow.

How to Ace FDA Food Label Compliance Review: Ensure Your Labels are Always Approved
To ace FDA food label compliance reviews and ensure your labels are always approved, tap into the power of automation. Automated quality control is a comprehensive solution for your compliance needs that ensures all of your food labels meet FDA label compliance review.

Overcome Your Content Challenges in Cosmetic Labeling to Meet FDA Requirements
Cosmetic manufacturers operate in a highly regulated industry, further complicating the labeling process. Not only do they have to consider branding and marketing guidelines, but they also have to focus on FDA cosmetic labeling requirements, as there are strict rules and regulations behind every cosmetic label.

How to Ease the Labeling Proofreading Process for Medical Devices
A medical device can range from the simplest household item found in everyone’s pantry, like a band-aid, to more complex technology like an x-ray machine. Regardless of its complexity, there is a constant amongst them all. They all need to be tested and approved to meet FDA requirements and compliance.

Ensure Your Labels Meet all FDA Drug Labeling Requirements with Automated Quality Control
For highly regulated industries like pharmaceuticals, following FDA drug labeling requirements is one of the most crucial aspects of the product lifecycle.
6 Ways Businesses Can Overcome Data Integrity Issues
In today’s digital age, a new threat presents itself to businesses that are heavily reliant on data. Many data-driven organizations rely on data integrity to be able to conduct their business operations without issues. These businesses must take great care to ensure the consistency, accuracy, validity, and safety of data.

Why Regulatory Affairs Needs Automated Proofreading Software
Now more than ever, the demand for safe and effective medical drugs is increasing on a global scale. This means that regulatory affairs teams are working overtime to interpret, apply, and communicate the guidelines received from governing bodies like the FDA and EMA when it comes to developing new drugs.

How FDA Regulatory Compliance Affects All Aspects of Manufacturing
There’s a saying: “Winning isn’t everything; It’s the only thing.” In business however, before you can win, you have to first comply with regulations.
Following FDA Data Integrity Guidelines Is Easier than You Think
Of note, the Food and Drug Administration (FDA) has official data integrity guidelines, out in full force as we speak. However, firms who are already following current Good Manufacturing Practices (cGMP) have nothing to worry about.

Why Automated Quality Control is Needed in Regulatory Affairs
Still in its relative infancy as a discipline, regulatory affairs was created to meet a pressing need, regardless of the industry in question.
FDA Break-Down: What is the FDA, FDA 21 CFR and How Do You Stay Compliant?
What is the FDA? The Food and Drug Administration is a government agency that operates under the United States Department of Health and Human Services. The FDA is responsible for protecting and ensuring public health in…

An Overview of ISO 9000 and ISO 9001 and Why They are Vital for Companies
Date: November, 2017 | Category: Compliance | Author: Marvin Magasura One way companies can ensure high-quality standards is by demonstrating to accredited organizations that they can fulfill specific QMS requirements.
Founded in 1947,…

Looking for Process Change? Think ISO 9000
Every company needs change, but in the midst of implementing it, only a few of them think about ISO 9000. It is mostly perceived as a purely technical standard that only specific industries have to deal with. But for the experienced business…
12 Ways to Reduce Data Integrity Risks for Regulated Industries
Date: December, 2016 | Category: Quality | Author: Mike Malz What Does Data Integrity Mean?
Data integrity refers to the fact that data must be reliable and accurate over its entire lifecycle. Data integrity and data security…

CMOs of Increasing Importance to Pharmaceutical Companies
Date: July, 2016 | Category: Compliance | Author: Reuben Malz The pharmaceutical marketplace has changed dramatically over the last few years as pharmaceutical companies are moving from centralized, internal production to single-source…

Having to Rework Label Artwork Again and Again to Stay in Compliance?
Date: April, 2016 | Category: Compliance | Author: Reuben Malz No matter what industry you are in there are always new regulations that impact the way you create labeling artwork. Staying compliant with regulatory labeling obligations…
![]()
GlobalVision is an intelligent inspection
platform that finds errors in your work
before they become problems.
Stay updated with GlobalVision by signing up for our newsletter.
Contact Us
GlobalVision HQ
16800 Route Trans-Canada
Montreal, QC, Canada
H9H 4M7
+1-514-624-4422
Company
Contact us
About
Partners
News & Events
Careers
Security
Privacy Settings
Subscribe to our newsletter.