Date: May, 2024 | Category: Proofreading | Author: Amanda De Luca
In the highly regulated and complex world of pharmaceuticals, accuracy and precision are paramount. Even minor oversights or errors in labeling, packaging, or documentation can lead to significant consequences from regulatory non-compliance issues such as sanctions and recalls, to potentially compromising consumer and patient safety.
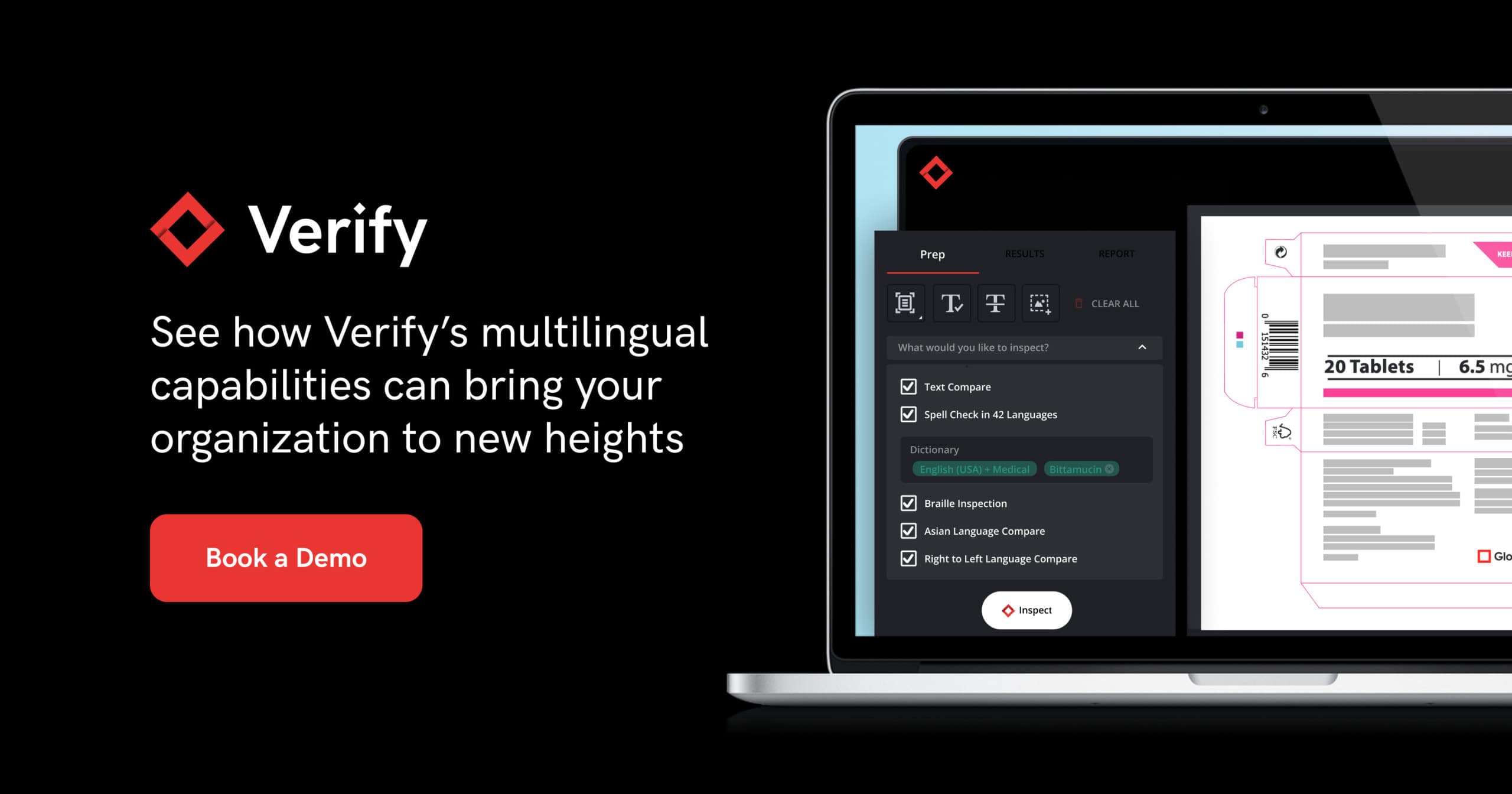
To navigate these challenges, leading pharmaceutical companies are increasingly leveraging advanced technologies like GlobalVision’s Verify to automate the proofreading and quality inspections of their critical content and labeling files.
In this blog post, we will explore 10 reasons why leading pharma companies are opting for automation with Verify and putting their trust in this market-leading software.
1. Ensures Regulatory Compliance
It’s no secret that health authorities such as the FDA in the United-States and the EMA in Europe impose stringent regulations and guidelines on pharmaceutical companies, making compliance a non-negotiable.
These companies operate within an environment of strict regulatory guidelines and any deviation from these standards throughout the drug development and packaging process, can lead to regulatory sanctions or even hefty fines—noting that this also applies to medical device companies.
Verify’s cloud-based automated proofreading tools are designed to detect any potential deviations or errors in documentation from text, graphics, barcode and even braille, thus ensuring that documentation and all critical content align with regulatory requirements, minimizing the risk of non-compliance and associated penalties. Key features built into the platform such as medical and custom dictionaries are there to streamline the workflows of regulatory professionals, and ultimately providing peace of mind when it comes to compliance.
2. Eliminates Human Error
Manual proofreading and quality inspections are prone to human error, especially when dealing with large volumes of documents which only increase with the size of the organization. Those tasked with this tedious process are often prone to fatigue due to long hours of reviewing text-heavy documents such as Instructions for Use (IFUs). This is where automated proofreading comes to play, setting out to eliminate the risk of human error in such processes.
Verify’s cloud-based automated proofreading tools significantly reduce the chances of oversights, ensuring that critical information is accurately reviewed and approved. As a result, the efficacy and accuracy of the proofreading process are drastically enhanced, offering a reliable solution for meticulous document scrutiny across pharmaceutical companies.
3. Accelerates Time-to-Market
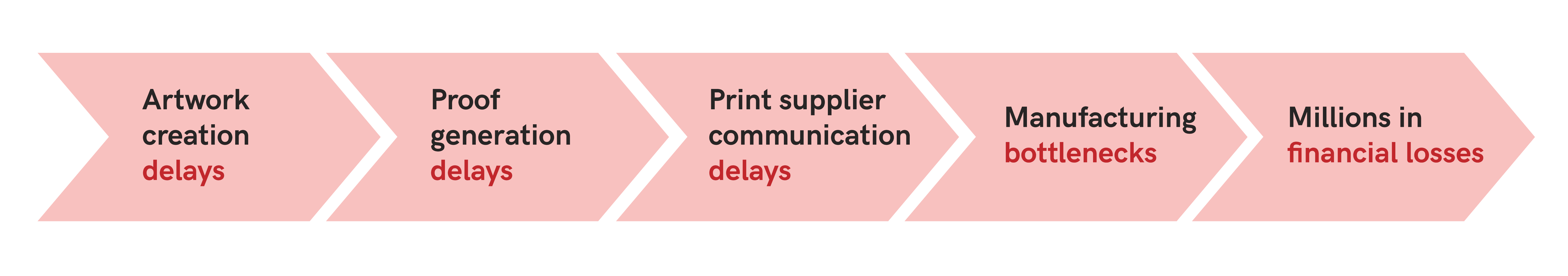
Experts suggest that delaying the commercialization or launch of a $150 million pharmaceutical product by just one month could lead to a sales shortfall exceeding $12 million. This is why life sciences enterprises cannot depend solely on existing full-time employees engaged in other duties to facilitate a product launch.
Verify’s automated proofreading software streamlines the proofreading process, reducing the time required for quality control and enabling companies to bring products to market faster without compromising on quality or accuracy.
By automating the proofreading process, Verify’s automated solution frees up valuable human resources within pharmaceutical companies allowing teams to focus on more strategic tasks, such as research and development, while the software aids in the meticulous task of quality control.
Thanks to this automation, the regulatory, labeling, and commercial processes within pharma are significantly optimized, resulting in a swifter time-to-market, which is key for capturing market share and maintaining a competitive edge in the market.
For perspective see what a top pharmaceutical company had to say about GlobalVision’s automated solutions, “Manually proofreading one document would take one hour to complete. Considering workloads of over 2 dozen document inspections weekly, manual checks would result in 24 hours of proofreading in a week. With GlobalVision, this task is completed within minutes.”
4. Helps with Brand Equity
Precision is crucial in pharmaceutical documentation, however this does not only apply to text but to all components of a document as well.
Pharmaceutical documentation often includes graphics such as logos, diagrams, and more. Automated proofreading, with its robust and comprehensive capabilities, is capable of inspecting these graphics for any discrepancies with pixel-to-pixel accuracy, ensuring that they meet quality standards and are correctly placed within the document without any errors.
What’s more, this precise graphics compare capability enables companies to better ensure brand equity by checking graphical components such as logos and images. By checking these graphics, companies can ensure that all brand guidelines are being followed and met and that products are reaching consumers at the highest quality, ensuring brand reputation and subsequently, brand loyalty.
With such precise and accurate graphics comparisons, pharmaceutical teams can be sure that not only is their text and spelling correct, but their artwork and complete product is accurate as well.
5. Eases Global Scalability
Pharma companies often operate globally and need to cater to diverse markets with different language requirements.
Automated proofreading tools such as Verify supports multilingual proofreading and checks spelling in 37 global languages which includes Medical and Custom dictionaries to cater to the unique terms and needs of individual companies. What’s more, Verify is able to find discrepancies in different scripts and alphabets such as Asian characters, Cyrillic, Greek, etc. and can also accurately inspect right-to-left languages such as Arabic and Hebrew.
This advanced capability not only aids in the enhanced accuracy of pharmaceutical products, it also makes scaling globally much easier for global enterprises with customers all over the world. In one quick inspection, companies can leverage the advanced language capabilities available to them in Verify and check for text and spelling differences, all within a seconds to minutes.
This allows companies to maintain consistency and accuracy across various languages and regions and enables them to scale more easily, meeting the needs of their customers worldwide.
6. Enhances Collaboration
Verify’s automated proofreading software facilitates collaboration among teams by providing a centralized platform for proofreading and quality inspections. This ensures that all stakeholders, from regulatory affairs to labeling and commercial teams, can review documents and share reports with relevant stakeholders, fostering better communication and alignment amongst teams and cross-functionally.
Also, the capacity to monitor modifications and uphold version control guarantees that every stakeholder has access to the latest information. This leads to better-informed choices and enhances the quality of documentation and subsequently, products.
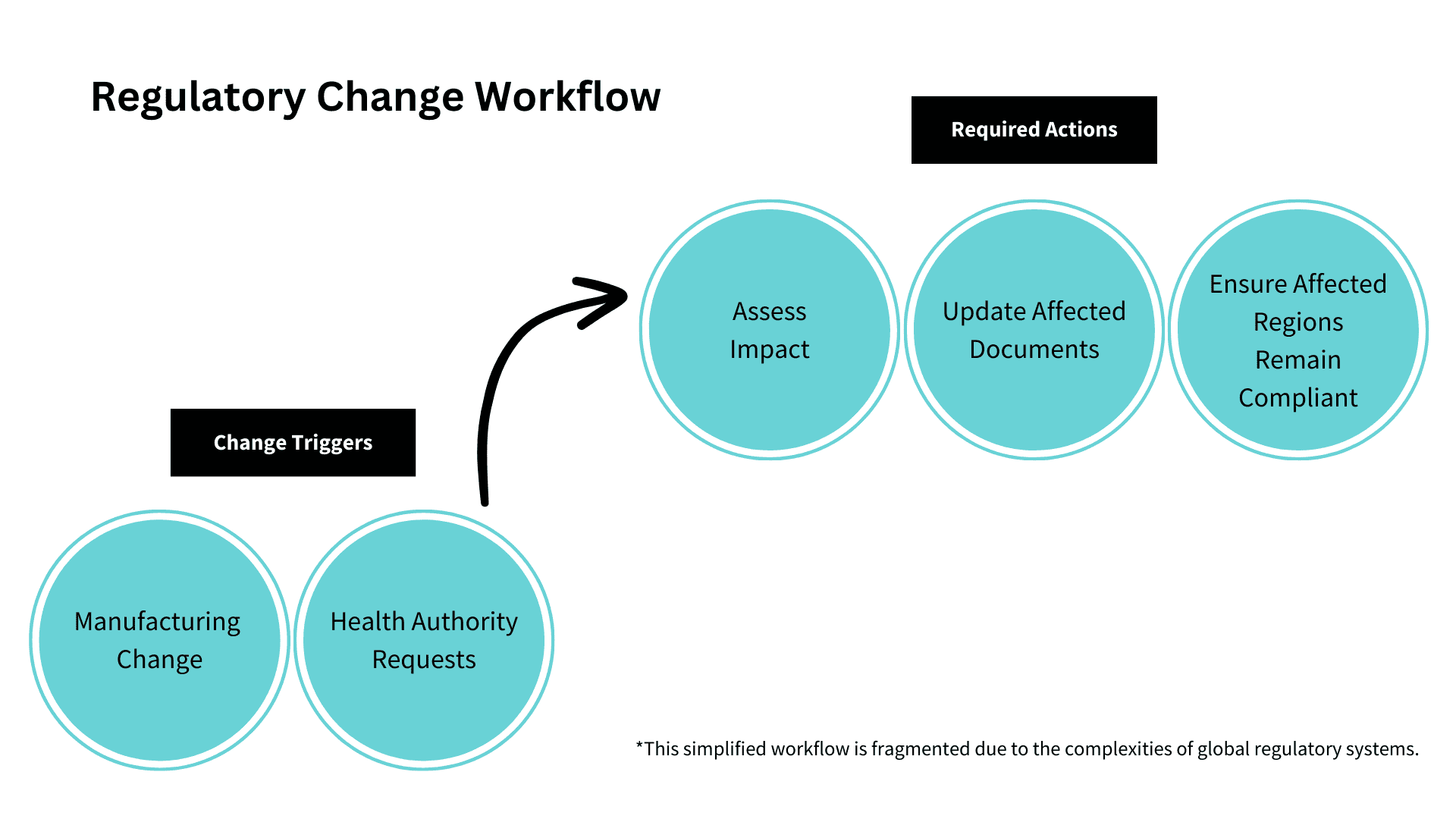
7. Minimizes Product Recalls
Product recalls can be detrimental to a pharmaceutical company’s reputation and finances.
For perspective, clinically important drug recalls occur approximately once per month in the United States while one of the top recall causes in pharmaceuticals is incorrect labeling.
Automated proofreading helps identify these potential issues early in the production process by pinpointing inconsistencies and deviations in critical documentation, allowing teams to catch errors on time and make the necessary adjustments before it’s too late. This enhances the accuracy and consistency of information, minimizing the risk of recalls due to documentation or labeling errors and safeguards both consumer safety and the company’s reputation.
8. Improves Cost Efficiency
Manual proofreading is not just time-consuming—it can also be expensive!
Hiring external proofreaders, especially when dealing with languages where native speakers are needed for the task, or simply allocating internal resources for this task incurs costs that add up over time. While implementing automated proofreading solutions may require an initial investment, the long-term cost savings and return on investment are substantial.
By reducing the need for manual proofreading and minimizing errors, Verify enhances cost efficiency in the pharmaceutical production process and is ultimately a solution that pays for itself through increased efficiency and error prevention.
9. Offers Comprehensive Reporting
For pharmaceutical companies, documentation is not just about producing critical documentation such as labels and inserts — it’s also about maintaining a detailed record of changes and approvals.
Verify, with its automated proofreading features, provides comprehensive reporting tools that track every step of the proofreading process, offering transparency and audit trails for regulatory purposes. This not only helps in inspection and revision workflows it also is a key competent in ensuring data integrity.
In fact, automated proofreading software is not just a form of error-detection software, GlobalVision’s Verify features an audit trail for compliance with FDA 21 CFR Part 11. The platform doesn’t just go over the document pixel-by-pixel or character-by-character to detect graphics and text differences, it also tracks parameter changes and log-ins, so data becomes “attributable,” one of the five principles of data integrity.
10. Adapts to Evolving Technologies
Verify prioritizes innovation and is constantly evolving through feature enhancements and regular updates.
As technology continues to advance, pharmaceutical companies must stay ahead of the curve. Verify, as GlobalVision’s newest and most innovative cloud-based, automated proofreading tool, is a future-ready solution that adapts to evolving technologies, ensuring that pharma companies can meet the challenges of an ever-changing industry.
The software is updated every quarter and also leverages the newest technologies such as machine learning and artificial intelligence. By embracing these technologies, Verify equips pharmaceutical companies with the latest and most cutting-edge proofreading tools, constantly setting the bar higher with each new software release.
Automate Your Proofreading Today
For the pharmaceutical industry, the benefits of automating proofreading processes with GlobalVision’s Verify are evident for top pharma companies striving for excellence in quality control.
From ensuring regulatory compliance to accelerating time-to-market, top pharma companies are choosing automation as a strategic investment in precision, efficiency, and overall operational excellence.
As the pharmaceutical world continues to evolve, automation through proofreading software becomes not just a choice, but a necessity for success in a highly competitive and regulated environment.
Learn more about Verify’s automated proofreading solutions by booking a demo today.