How AI Proofreading Will Power the Future of Pharma
Date: November, 2023 | Category: Proofreading | Author: Gabriella Naguib
In the rapidly evolving pharmaceutical landscape, accuracy, compliance, and speed of regulatory processes are key. Traditional proofreading and compliance review methods for documentation and packaging, labels, and more have proven to be time-consuming and error-prone, often leading to delays to market, financial losses, non-compliance issues, and consumer safety risks.
The introduction and recent rise of artificial Intelligence (AI) and automation proofreading technology allow pharmaceutical companies to reap significant competitive advantages by streamlining workflows, reducing errors, and getting to market faster for often life-saving drugs. Consequently, these technologies also protect consumers and patients from potential adverse effects or improper use.
Pharmaceutical companies that are ahead of this technological curve are already benefiting from the introduction of AI and automation in their workflows. Heightened accuracy, compliance, and speed are only some of the advantages they reap, but as the technology continues to grow and develop, regulated industries–especially pharma–can expect to continue to benefit from such technologies in the future.
What is Artificial Intelligence (AI)?
AI focuses on creating intelligent systems capable of simulating human-like intelligence. Machine learning is one of the most popular techniques used in AI, which allows computers to learn from data, recognize patterns, and make decisions. These capabilities enable AI systems to adapt and enhance their performance over time.
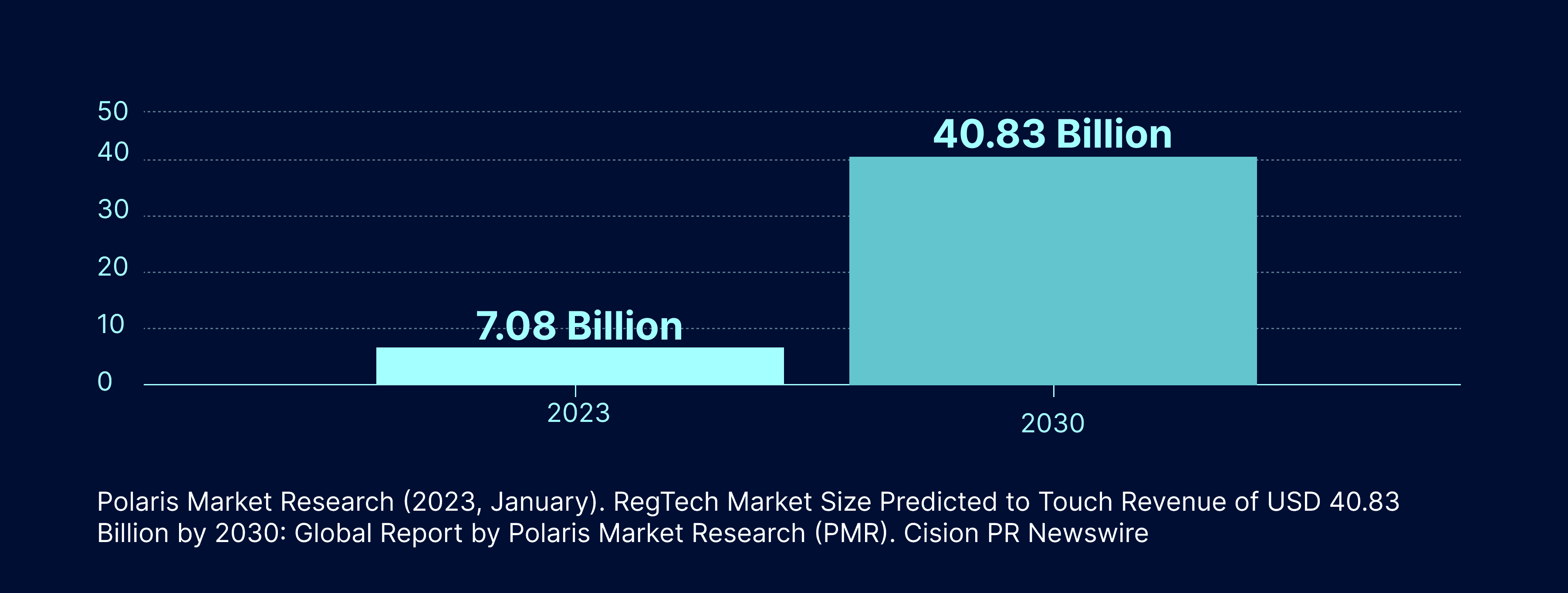
The field of Regulatory Affairs within the Life Sciences industry is currently undergoing a significant transformation, thanks to the emergence of AI-powered technologies. These technologies are currently being utilized in Regulatory Technology (RegTech) to improve end-user performance. However, the potential for future use cases is vast. Although the use of AI in this field is currently relatively new, the RegTech industry is expected to grow from 7.08 billion in 2023 to 40.83 billion by 2030, and is well-positioned to leverage the power of AI.
How Pharma Regulatory Leverages AI Proofreading
A G2 study from June 2023 reveals that artificial intelligence and machine learning are the primary technologies used for compliance procedures, assisting in risk assessment, crisis management, and predictive analytics.
A prime example of how regulatory professionals in the industry leverage these technologies to automate compliance procedures is through AI-powered solutions. One emerging and innovative cloud-based proofreading software that is changing the way regulatory businesses are using automation in their quality assurance processes is, Verify.
Verify is the newest and most innovative proofreading software from GlobalVision that harnesses the power of AI to conduct accurate and thorough inspections of regulatory documentation. It allows regulatory users to perform quality checks across critical content such as digital packaging assets and labels by automating key tasks such as text inspection with character for character precision.
A key component of these quality checks is the ability to inspect documents whether they contain live text or not. To facilitate this, Verify has built-in OCR (Optical Character Recognition) capabilities that are driven by artificial intelligence. By leveraging these technologies, regulatory affairs professionals can handle reviews such as regulatory submissions and labeling inspections with complete ease, in record time.
The Advantage of AI Proofreading
GlobalVision’s independent market research has recently shown that AI-powered technologies help pharmaceutical companies get their products to market faster. Our survey results revealed that 89% of our regulatory survey respondents said that using our AI-powered automated proofreading solutions significantly reduced their compliance review process, allowing them to deliver products to market faster.

These findings provide strong support for the same claim through a customer case study involving a large pharmaceutical enterprise. The case study revealed that prior to implementing our AI-powered automated proofreading solution, the company had difficulties getting their products to market ahead of competitors, which ultimately hindered their ability to capture any market share in certain instances.
Elevating Core Technology with AI Proofreading
Beyond Optical Character Recognition (OCR), the industry is delving into a realm where AI plays a pivotal role throughout the entire content management and review process. In the near future, substantial improvements driven by machine learning will allow RegTech solutions to surpass the confines of rule-based systems that have previously governed SaaS proofreading algorithms.
This new ‘AI era’ presents an unprecedented opportunity. Tech companies can now “teach” their algorithms how to comprehend documents by continuously feeding them vast volumes of data over time. The result? Faster, better, more streamlined workflows and quality control processes that ultimately lead to more accurate inspections and content.
What’s more, as time passes, it is inevitable that these solutions will further improve and transform into “intelligent technology” at their core, designed with increasing precision to meet the unique needs of each and every customer.
AI to Simplify User Experience
With this strategic shift and AI at the forefront, regulatory users can expect even further automation for a multitude of tasks throughout the content management workflow, without compromising their trust in these technologies.
The automation of said tasks will be infused with a deeper, more human understanding of the documents that regulatory professionals review on a daily basis.
Imagine a level of understanding that encompasses simple nuances such as page numbers, document types (IFU, QRD, SPL templates, inserts, cartons), and structural components, including prescribing information highlights, tables of content, full prescribing information, forms, strengths, warning statements, dosages, and even the correctness of dosage units.
With this technology constantly expanding and becoming more common in regulatory workflows, users can look forward to a transformative end result that enhances their workflow and instills confidence in the technology they rely on daily.
What this means is as AI-driven proofreading technology advances, it promises faster and more accurate error detection. AI’s rapid analysis of large volumes of text will enhance productivity and ensure quicker turnaround times. The improving accuracy stems from machine learning algorithms that understand context, offering nuanced suggestions that will improve the overall quality of content.
Moreover, AI’s adaptability will allow for personalized proofreading, aligning with individual organizations needs and goals. This evolving technology can be used to not only detect text errors but beyond that, including graphics, formatting, color, braille, and barcode errors providing relevant insights catered to each specific business.
The final benefit to end-users is the seamless and highly efficient automation of numerous quality control tasks. In essence, AI-enhanced proofreading will transform and streamline the entire revision process, fostering comprehensive, efficient, high-quality content across various workflows.
AI Proofreading for Medical Terms
Going a step further, the first step into this journey could involve AI applications to detect and analyze medical terms by harnessing the power of machine learning. This new approach would revolutionize how medical dictionaries are managed and updated, making for a more sustainable and effortless approach for SaaS providers, as opposed to constantly cross-referencing to a medical dictionary such as Stedman’s Medical Dictionary.
Users would benefit by being able to rely on a continuously updated AI engine to detect and inspect medical terms and eliminate the risk of non-compliance issues occurring. It would also eliminate the need for tedious and time-consuming manual checks of medical terms that aren’t included in a specific dictionary.
AI Proofreading for Allergens
Much like medical terms, allergen statements is another area of exploration that this exciting new field presents. With the assistance of machine learning, solutions could flag allergen statements and analyze their components at the same level as a human reviewer. This simple addition promises to automate compliance reviews, eliminate laborious manual checks, and contribute to the elimination of recalls and non-compliance sanctions.
The applications of AI for medical terms and allergen statement reviews could potentially help users meet FDA requirements in those areas as well as the requirements of other global health authorities.
AI Proofreading in the Future
In conclusion, AI and machine learning hold vast potential for widespread adoption in regulated industries and the possibilities are endless.
While the level of trust in AI is still evolving, the role of artificial intelligence in proofreading will continue to grow steadily. And while human intelligence remains an indispensable asset in the revision and quality control process, for the foreseeable future in regulated industries, AI will act as a trusted assistant and an added layer of inspection when dealing with critical and regulatory documentation.
Want to learn more about how AI can help elevate your regulatory business? Download our Regulatory Insights Report: The Rise of Automation Technology in Regulatory Affairs and take a deeper dive into more compelling data from regulatory professionals and their use of technology.
If you would like to start experiencing the time-saving and compliance enhancing powers of AI and Verify, get started today with a Free Trial.
*****
Are you interested in being part of the future of our AI-powered technology? We are looking for Verify customers and prospects to participate in our beta group for new AI technologies – our Innovation Advisory Board. Have your voice heard and sign up here.