Compliance in Medicine Post Brexit: Everything You Need to Know
Date: November, 2023 | Category: Compliance | Author: Hana Trokic
As we continue to process the historic decision of the United Kingdom to exit the European Union, commonly known as Brexit, its far-reaching implications continue to unfold across various sectors. In terms of healthcare and pharmaceuticals, the repercussions are expected to be particularly noticeable and profound.
After years of negotiations and political turbulence, Brexit, which officially came into effect on January 31, 2020, has cast a shadow of uncertainty over the pharmaceutical industry in the UK and the EU.
The intricate web of Brexit’s impact on medicine compliance, pharmaceutical trade, and regulatory frameworks is yet to be fully understood but one thing is for certain – it’s multifaceted and intertwines issues of access to medicines, the integrity of pharmaceutical supply chains, and the development and approval of new drugs.
From supply chain disruptions stemming from customs and border checks to evolving drug approval processes as the UK establishes its own regulatory path, we look at how this shift is reshaping the landscape of medicine compliance and pharmaceuticals in the UK and EU.
By navigating the complexities and challenges faced by pharmaceutical companies, healthcare providers, and patients, read on to learn more about the strategies and adaptations emerging in the post-Brexit era to ensure the continued delivery of safe and effective medicines to those who rely on them.
Medicine Compliance in the UK Post Brexit

One of the immediate concerns that surfaced with Brexit was its impact on medicine compliance. Prior to Brexit, the European Medicines Agency (EMA) played a central role in regulating and approving medicines across the EU, including the UK. However, with the UK’s departure, the country’s Medicines and Healthcare Products Regulatory Agency (MHRA) became a stand-alone body as it ceased to be part of the European system of approval.
This transition led to a series of challenges, including the need for pharmaceutical companies to duplicate their efforts by submitting separate pharmaceutical applications to both the EMA and MHRA for approval.
This duplication not only increased regulatory burdens but also posed questions about the consistency of medicine approvals and what compliance is in medicine post Brexit.
Patients and healthcare providers needed assurance that the medicines they relied on remained safe and effective. Consequently, many pharmaceutical companies undertook the arduous task of adapting to these new compliance requirements, investing time and resources to ensure a seamless transition.
The Pharmaceutical Trade Landscape
The pharmaceutical industry relies heavily on global supply chains, making it particularly vulnerable to disruptions caused by Brexit. The imposition of customs and border checks created bottlenecks and delays, affecting the timely delivery of vital medicines to patients on both sides of the English Channel.
Pharmaceutical companies were forced to reevaluate their supply chain strategies, with some choosing to stockpile medicines to mitigate potential shortages. However, stockpiling is not a sustainable long-term solution and comes with its own set of challenges, including increased storage costs and the risk of medicines expiring before use.
Moreover, the UK’s departure from the EU single market and customs union introduced new regulatory barriers to pharmaceutical trade and new medicine compliance rules. Companies had to navigate complex rules of origin, tariffs, and customs procedures, all of which added to the cost and complexity of doing business.
To address these challenges, some pharmaceutical firms explored the possibility of relocating parts of their operations to the EU to maintain access to the single market. This then leads to the potential of further economic consequences for the UK as valuable companies and jobs are no longer located on English territory.
Parallel Importation
Another challenge that arose was the stricter regulations regarding parallel importation.
Parallel importation refers to the practice of importing pharmaceutical products from one country to another within the European Market Area (EMA) to take advantage of price disparities. After Brexit, the UK is no longer part of the EU single market, meaning parallel importation became significantly harder.
They are now subject to stricter medicine compliance regulations and have to apply for licenses to import goods that could have easily been shipped throughout the Euro Zone prior to Brexit. In the future, this could affect the availability of certain medicines and their cost in the UK.
Customs and Supply Chain Disruptions
While exiting the EU made certain things easier for the UK, it certainly also introduced many more regulations and complications with regard to trade between the two European territories.
Brexit introduced customs checks and border controls between the UK and the EU, which has been shown to lead to supply chain disruptions for pharmaceutical companies. Delays in transporting raw materials and finished products can, and to an extent, have impacted the availability of medicines on both sides of the border.
Pharmaceutical companies have had to adjust their supply chain strategies to mitigate these challenges, but the potential for even more disruptions remains a large concern.
The Evolution of Drug Approval Processes

Brexit also prompted a shift in the landscape of drug approval processes.
The UK now had the opportunity to develop its own medicine compliance regulatory framework, separate from the EMA. While this could potentially streamline decision-making and reduce bureaucracy, it raised concerns about the duplication of efforts and the potential for divergence in standards between the UK and the EU.
To mitigate these risks, the UK and EU agreed to a medicine compliance regulatory cooperation framework, ensuring continued information sharing and collaboration on drug approvals. However, challenges remain as the two entities adapt to their new roles and responsibilities in this post-Brexit era.
Pharmacovigilance and Safety Reporting
Pharmacovigilance, the monitoring of the safety of pharmaceutical products once they are on the market, is a crucial part of the pharmaceutical product lifecycle, and another area affected by Brexit.
The UK and the EU now have separate pharmacovigilance systems, leading to the need for separate safety reporting and data management processes. Ensuring seamless cooperation and information exchange between these systems is crucial to maintaining patient safety and medicine compliance.
Yet, with the additional regulations being placed after Brexit and the further complication of the entire process, the chance of errors occurring is higher, therefore, the need to mitigate them is also a larger concern.
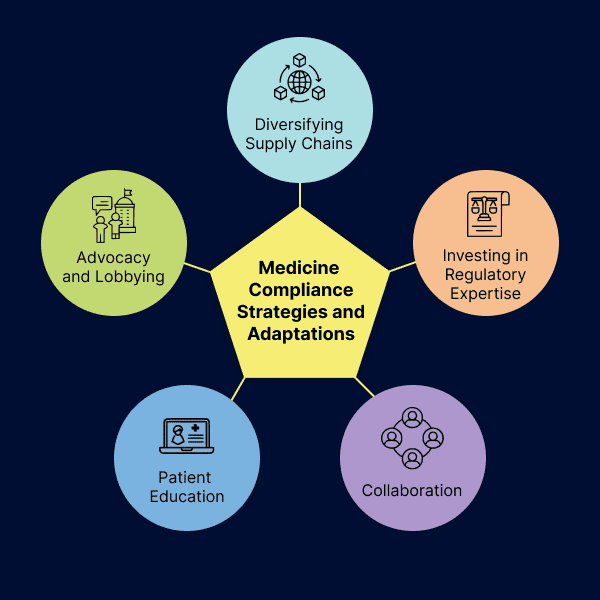
Medicine Compliance Strategies and Adaptations
In the face of these medicine compliance challenges, pharmaceutical companies, healthcare providers, and patients have to quickly adapt to the post-Brexit reality.
Strategies to ensure the continued delivery of safe and effective medicines include:
- Diversifying Supply Chains: Many pharmaceutical companies are diversifying their supply chains, reducing their reliance on a single source for critical components and ingredients. This strategy enhances resilience and reduces the risk of disruptions.
- Investing in Regulatory Expertise: Companies are investing in regulatory expertise to navigate the complexities of dual regulatory systems. This includes hiring regulatory professionals and consultants to manage compliance effectively.
- Collaboration: Collaboration between pharmaceutical companies, regulatory authorities, and healthcare providers remains crucial. Information sharing and cooperation can help address challenges and ensure a smoother transition.
- Patient Education: Patients are being educated about potential changes in the availability of their medicines and are encouraged to consult with healthcare providers to explore alternative treatments or solutions.
- Advocacy and Lobbying: Pharmaceutical industry associations are actively engaging with policymakers to advocate for policies that support the smooth flow of medicines and reduce trade barriers.
Document Comparison Software: The Ultimate Post-Brexit Strategy
Along with the strategies mentioned above, document comparison software offers significant advantages in the domains of medicine compliance, pharmaceutical trade, and post-Brexit regulatory adherence.
It aids medical facilities in maintaining – medicine compliance by ensuring the accuracy of regulatory content, supporting version control, and facilitating audit readiness. In the pharmaceutical sector, in particular, it helps companies uphold product labeling and manufacturing standards, ensuring compliance with international trade regulations and reducing the risk of trade disruptions.
Moreover, document comparison software plays a pivotal role in adapting to post-Brexit medicine regulations by ensuring legal compliance, monitoring trade agreements, and mitigating risks associated with regulatory changes. Its ability to swiftly identify inconsistencies and compliance gaps makes it an invaluable tool for navigating these complex and highly regulated industries.
How to Ensure Medicine Compliance With Document Comparison Software?
Here’s how document comparison software can help in post-Brexit medicine compliance regulatory adherence:
- Regulatory and Medicine Compliance: Document comparison software can assist in ensuring that medical documents, such as patient records, compliance documents, and regulatory submissions, are up to date and aligned with the latest healthcare regulations. This is crucial, as non-compliance can lead to legal issues and harm patient care.
- Version Control: The software helps in managing version control of critical documents, making sure that the latest and approved versions of documents are being used by quality assurance teams. This ensures that pharmaceutical documentation, packaging, and labeling, remain compliant with evolving regulations.
- Audit and Inspection Support: The pharmaceutical industry is regularly audited and inspected. Document comparison software can help in preparing for audits by identifying any inconsistencies or non-compliance issues in advance, reducing the risk of compliance violations.
- Product Labeling and Packaging: In the pharmaceutical industry, accurate labeling and packaging of products are essential. Document comparison software can ensure that product labels and packaging materials comply with international trade regulations, reducing the risk of product recalls or trade disruptions.
- Batch Records and Manufacturing: Pharmaceutical companies can use document comparison software to ensure that batch records and manufacturing processes adhere to quality and safety standards, which are critical for successful international trade.
- Regulatory Submissions: Companies can use the software to compare regulatory submissions before and after Brexit to ensure that they are in compliance with the new post-Brexit regulations. This reduces the risk of regulatory issues and trade disruptions.
- Legal Compliance: Document comparison software can be used to compare legal documents before and after Brexit, ensuring that new documentation is updated and accurate and that companies are aware of and adhere to the new regulations and trade agreements that have come into effect.
- Trade Agreements: The software can help companies in the pharmaceutical and medical industries ensure that they are in compliance with any new trade agreements or tariffs that have been established as a result of Brexit.
- Risk Mitigation: By regularly comparing documents with the latest, updated versions, organizations can identify and address compliance gaps or inconsistencies promptly, reducing the risk of legal issues and trade disruptions.
The Future of Pharma and Brexit
Brexit has undeniably cast a shadow of uncertainty over the pharmaceutical industry, but it has also spurred innovation and adaptation. As pharmaceutical companies, healthcare providers, and patients navigate the complexities of medicine compliance, supply chain disruptions, and evolving regulatory frameworks, the resilience and determination of the industry to ensure access to safe and effective medicines remain unwavering.
While the full implications of Brexit on healthcare and pharmaceuticals are still unfolding, the commitment to safeguarding the health and well-being of patients on both sides of the English Channel remains of the highest importance.
The post-Brexit era presents an opportunity for the industry to demonstrate its adaptability and dedication to delivering vital medicines to those who rely on them. One way in which they can ensure this is through the implementation of efficient, streamlined, and error-free processes.
Implementing document comparison software in regulatory and quality assurance processes can help maintain medicine compliance, ensuring that documentation and pharmaceutical processes adhere to evolving healthcare regulations with complete ease while giving regulatory professionals peace of mind that no errors are slipping through.
Take a step in the right direction by setting up proper processes through document comparison software. Visit our Demo Center to learn more about our products at your own leisure and see how this technology can revolutionize your everyday business and regulatory practices.