Manual vs. Automated Proofreading: A Comprehensive Guide
Date: December, 2023 | Category: Proofreading | Author: Hana Trokic
The way we communicate with others is essential. How we express ourselves through different outlets is crucial in getting our exact message across exactly how we intend it to.
The same can be said for businesses, as companies spend immense amounts of time, money, and resources ensuring they communicate their brand seamlessly to every consumer. Yet, a brand’s image isn’t just a mission statement or a slogan. Behind each brand is a mountain of content and documentation – from packaging and labeling to legal, digital and printed content.
It begs the question. Manual or automated proofreading? Which method will better ensure brand integrity when products go out to market?
What is Manual Proofreading?
Manual proofreading refers to the process of carefully reviewing and checking documents or content by human experts to ensure accuracy, compliance, and adherence to established standards and regulations. This process involves meticulous examination of the content for errors, inconsistencies, or any deviations from the regulatory requirements.
The Risks of Manual Proofreading
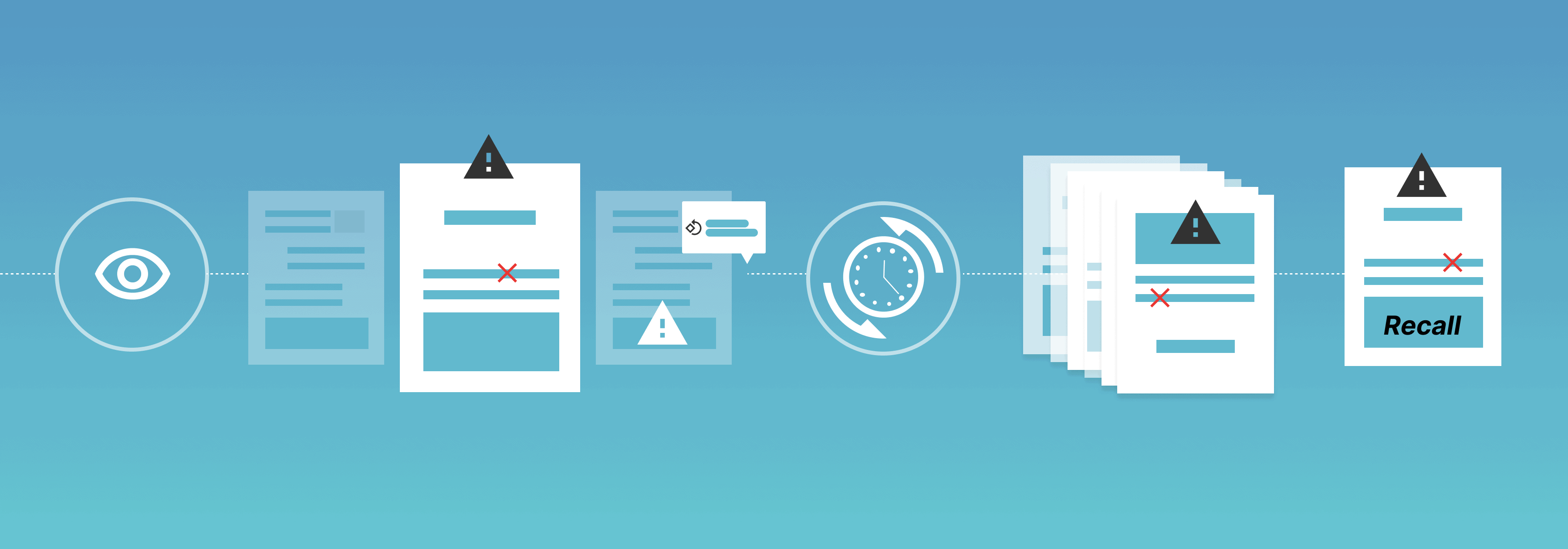
Manual proofreading implies human proofreading of all documentation before it goes out to print.
With an endless stream of documentation that needs to be constantly reviewed, manual proofreading is not without its challenges. Not only is it time-consuming, but the chance of human error is high, while some mistakes can result in significant consequences for companies, be it financial, legal, or brand-wise.
Other companies face the challenge of having documents that simply cannot be manually proofread since errors in barcodes, fine print, and graphics are often far too difficult for the human eye to detect.
While manual proofreading will always play an essential role in many quality assurance processes, it is important to note its many risks if used as a primary method of proofreading.
Some of the risks associated with manual proofreading include:
- Human Error: Humans are prone to errors, and manual proofreading relies on human judgment. Mistakes, oversights, or misinterpretations may occur during the manual review process, potentially leading to inaccuracies in the verification results.
- Scalability Issues: Manual proofreading can be time-consuming, making it challenging to scale the process efficiently, especially when dealing with large volumes of documentation. This can result in delays and increased costs.
- Limited Coverage: Due to time constraints, manual proofreading may only cover a subset of the data or content, leaving some areas unchecked. This limited coverage increases the risk of overlooking errors or non-compliance issues.
- Resource Intensiveness: Manual proofreading requires human resources, which can be expensive and time-consuming. Organizations may need to allocate significant manpower to ensure thorough verification, impacting overall efficiency.
- Consistency Challenges: Ensuring consistent application of verification criteria across different reviewers can be challenging. Variability in interpretation and decision-making among reviewers may lead to inconsistencies in the verification process.
What’s more, manual proofreading is not highly accurate when dealing with graphics, color deviations, barcodes, or braille. Unless the mistakes are evident, the human eye cannot detect minor discrepancies that otherwise compromise the integrity of the overall design, packaging, label, and so on.
To stay competitive in today’s market, companies simply cannot afford the setbacks of manual proofreading. This is why many modern-day businesses turn to automated technologies to help increase the speed, accuracy, and efficiency of their revision workflows.
Want to learn more about the risky business of manual proofreading? Read our blog post here.
Automated Quality Control as a Proofreading Solution
Automated quality control and proofreading stands out as a perfect proofreading solution for businesses who wish to reduce the risks and setbacks that come from manual proofreading.
This technological innovation provides better proofreading results and enables complete and comprehensive inspections of critical content and documentation.
In essence, automated quality control and proofreading works by comparing two documents to find discrepancies between the two. The software conducts comprehensive inspections and proofreads everything from text, color, graphics, barcodes, braille, and more. Inspections are completed exponentially faster than manual proofreadings and a task that used to take hours or days is now reduced to only a few minutes.
The Benefits of Automated Proofreading
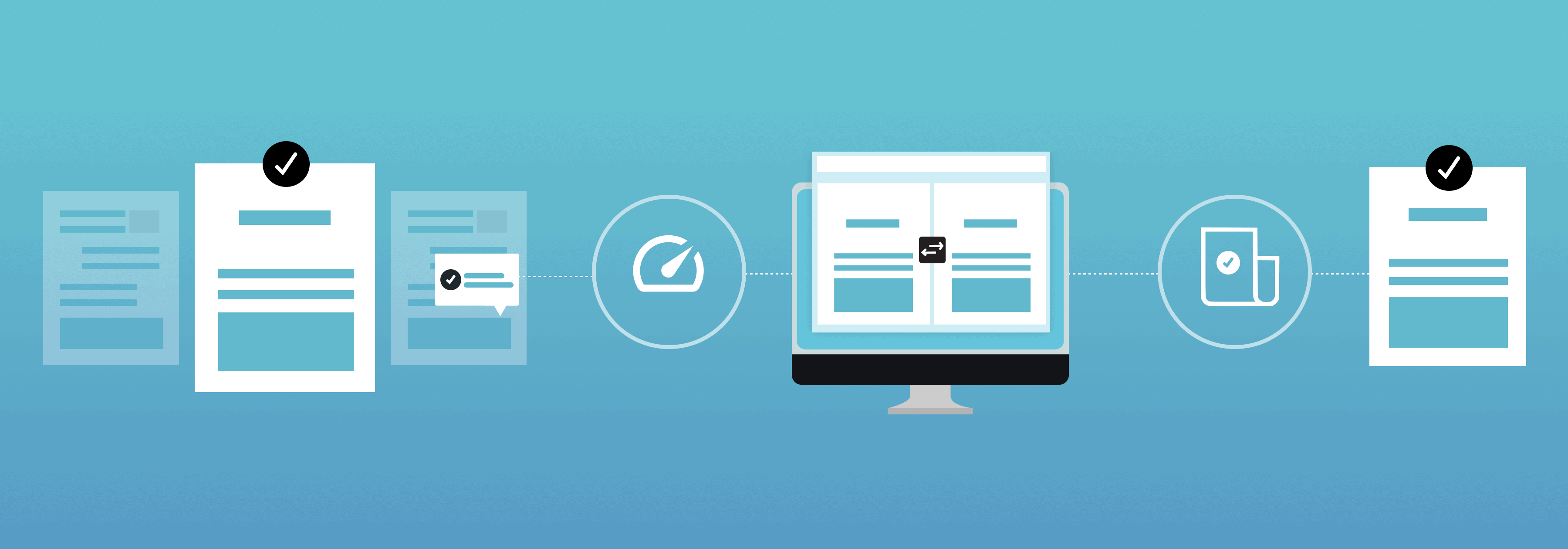
Automated quality control and proofreading offers several advantages across various industries and processes.
Some key benefits include:
- Speed and Efficiency: Automated quality control can proofread and process large volumes of data or content at high speeds, reducing the time required for reviews compared to manual methods. Automated systems can also perform repetitive tasks consistently and without breaks, contributing to increased efficiency.
- Consistency: Automated proofreading ensures consistent application of predefined criteria or rules, eliminating variations in judgment that can occur with different human reviewers. Consistency is crucial in maintaining product or service quality and compliance with standards.
- Scalability: Automated systems can easily scale to handle growing volumes of content. The scalability of automated processes allows organizations to maintain quality even as their operations expand.
- Reduced Human Error: Automated systems are not prone to the same types of errors that humans might make, such as overlooking details, miscalculations, or fatigue-related mistakes. This reduction in human error contributes to higher accuracy and reliability in quality control processes.
- Data Analysis and Reporting: Automated quality control and proofreading systems can generate detailed reports and analytics, providing insights into trends, patterns, and areas for improvement. Data analysis capabilities help organizations make informed decisions about their processes and quality improvement strategies.
- Cost-Effectiveness: While there may be upfront costs associated with implementing automated quality control and proofreading software, they often lead to cost savings in the long run by reducing the need for manual labor and minimizing the risk of errors that could result in costly consequences.
- Adaptability and Flexibility: Automated systems can be easily adapted to changes in criteria or regulations, making them flexible in addressing evolving business needs. This adaptability allows organizations to stay agile and responsive to shifting requirements.
- Integration with Other Systems: Automated quality control and proofreading systems can often integrate seamlessly with other technologies and systems, streamlining workflows and improving overall process efficiency.
To learn more about how automation can help your organization avoid costly mistakes, read Manual Proofreading Errors: How to Stop Label Recalls in Your Supply Chain
Automated Proofreading for Error-Free Content
One area where companies can largely benefit from automated quality control and proofreading is packaging and labeling. Pristine, error-free packaging and labeling not only increases customer loyalty to your brand, it’s also a necessity, regardless of industry.
It ensures that companies meet strict regulations from regulatory agencies such as the FDA for the United States, the MHRA for the United Kingdom, and EFSA for the European Union to name a few. This helps avoid potential compliance issues that lead to severe consequences.
Error-Free Packaging and Labeling
A packaging or label error doesn’t have to be fraudulent to be costly. In fact, a great deal of mistakes are simply caused by human error. Market research indicates that 60% of product recalls are caused by human oversights. 
Human errors can easily be introduced and missed in content and only reinforce the need for added layers of reassurance, such as automated quality control and proofreading software.
Performing label and packaging inspections at each stage of the revision cycle reduces the risk of a recall and the number of required revision cycles, so products get to market faster, without any errors.
Consequences of Label Errors
Taking the food and beverage industry as an example, packaging must contain what the label entails. If the FDA has reason to believe packaging is inaccurate, that it falsely claims the contents of the product inside, imports can be detained without physical examination. The product effectively gets prevented from being distributed and ultimately sold.
If a manufacturer is found to be guilty of breaching FDA regulations and compliance, the FDA may issue a warning. In the event the manufacturer does not comply and correct the issue, the FDA takes additional legal action to ensure the product gets removed from the market, without it being permitted to return until the issue has been corrected. Criminal fines and even prosecution may result depending on the infraction.
In the case of the pharmaceutical industry, which is also under the purview of the FDA and whose customers depend on packaging for accurate dosage information, it’s easy to see why penalties need to be especially severe. A single misplaced period can have huge negative ramifications.
Find out more about creating error-free content by reading Why Error-Free Packaging and Labeling Is a Must
Tips for Creating Error-Free Content
There are many different components involved when creating content for regulated industries, which includes packaging and labeling. To help ensure that you create error-free content and reduce the risk of dealing with a product recall, it’s always helpful to implement some best practices.
Proofreading Starts With Up-to-Date PDFs
Throughout a proofreading cycle, a PDF can go through many revisions before getting approved. Files can also be stored across different network shares or even on an individual’s local computer. It’s key to ensure you know where to find the most up-to-date PDF. By doing so, businesses will avoid sending the wrong PDF to print.
Check Barcodes Early
It’s common for graphic artists to put placeholders for barcodes to show where the final barcodes will be positioned on a file. An FPO symbol will often be placed on top of a barcode which stands for “For Position Only.” This is often missed when the print runs, resulting in the correct barcode not appearing.
It’s recommended to check barcodes on proofs which can be done through automated quality control and proofreading for checking barcodes or printing out the proofs and using a handheld verifier.
Proofread Printer Proof Prior to Approval
It’s easy enough to give a quick glance over artwork files and tell the printer to go ahead with the job, but this is not enough to ensure no mistakes have slipped through.
Conducting a thorough proofreading of files to check for errors in spelling, color, barcodes, and other content on a proof is essential. One way to do this type of check is to do a line-by-line visual comparison.
Standardize Design Tools
Do you know what versions of Adobe Illustrator your suppliers are using? Chances are they are all using different versions, which equals different functionalities and feature sets.
Ensuring everyone is on the same version means everyone is viewing the same file and that you have repeatable results. This also ensures there won’t be any unintended changes due to differences in software.
Continuous Quality and Improvement
Continuous testing in software is the new approach when developing products. Most software products today will have some level of automated testing completed to check against expected results. This helps to continuously test code and ensure quality throughout the development process.
Read 6 Tips for Creating Error-Free Packaging for more information about how to create perfect content every time.
There’s no reason why this methodology can’t be applied to any business’s revision cycle, regardless of industry. Using tools to perform routine tasks like preflight and PDF comparison can enable automation on a continuous basis to catch errors in files early on before products go out to market.
By implementing these best practices, businesses will see an immediate reduction in errors and an overall improvement in quality.
Want to learn more about automated quality control. Read our guide An Introduction to Automated Quality Control
Automate Your Manual Processes
To keep up with modern trends and ever-growing consumer demands, manual proofreading is simply not an adequate solution to allow for proper functions within a company’s quality assurance workflow.
While traditionally manual proofreading was the only method used to revise critical documentation, modern-day technology and innovations have allowed for more intelligent, more efficient solutions to get the job done.
With endless benefits and countless advantages to your workflow, automated quality control and proofreading is the leading solution for companies who want to revolutionize their revision processes.
To ensure the complete integrity and accuracy of all files, request a demo of GlobalVision today, and learn about all the advantages it can bring to your company while modernizing your current practices.
———————————————————————————————————————————————————
Related Articles:
- Manual Proofreading Errors: How to Stop Label Recalls in Your Supply Chain
- Why Error-Free Packaging Labeling Is a Must
- An Introduction to Automated Quality Control
- Risks of Manual Verification as a Proofreading Method
- How Automated Proofreading Is Replacing Obsolete Diff Checker Tools
- Proofreading Test: Comparing Results in Microsoft Word, Grammarly, and GlobalVision