Revolutionizing Braille Inspection: Introducing the Upgraded GVD Braille Module
Date: December, 2023 | Category: Compliance | Author: Hana Trokic
In regulated industries, where adherence to standards and accessibility is crucial, Braille is a vital component of any product’s packaging or labeling to foster compliance and inclusivity.
Braille, a universally accepted system of writing designed for individuals with visual impairments, is critical in ensuring that information, particularly in industries subject to strict regulations such as pharmaceuticals, is universally accessible.
This code not only empowers individuals with visual disabilities by providing them with independent access to written content, but it also aligns with non-discrimination principles mandated by regulatory agencies.
From a quality control perspective, Braille Inspection and accuracy play an important role as Braille is mandatory on pharmaceutical packaging all across Europe, and is strongly recommended by the FDA in Western markets, while other regions such as the ASEAN markets are working to heighten inclusivity by implementing braille requirements on packaging soon.
For regulatory industries to ensure the complete accuracy of their products, they need to ensure comprehensive revisions and inspections of their content. This includes text, graphics, barcodes, colors, and of course, braille for an all-encompassing quality control process. One way to ensure this accuracy is through modern-day technological solutions and innovations that ease these critical yet demanding revision tasks.
The New GVD Braille Module
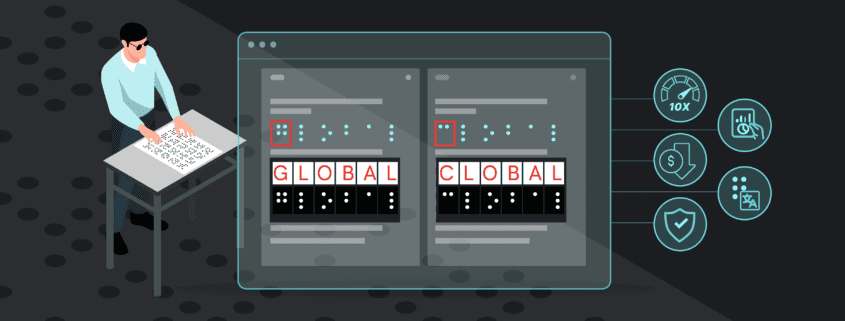
One such innovation that stands out for compliance reviews is the newest upgrade from GlobalVision—the GVD Braille Module. This new braille inspection technology sets a new standard for fast, accurate, and comprehensive braille inspections.
The GVD Braille Module allows regulated industries such as pharmaceuticals and print and packaging to automate braille height inspections with unparalleled granularity and speed, providing braille compliance data for each individual braille dot. This market-disrupting product brings unprecedented braille height detail, as well as meticulous braille translations to ensure complete accuracy and compliance with industry regulations across all printed assets.
This advanced technology is not just an upgrade in compliance inspections, it’s a breakthrough in braille inspection.
Here’s what to look forward to with the newest GVD Braille Module:
- 10x faster inspections compared to traditional softwares
- Example: DotScan on average takes 50 mins to complete a full Press sheet Braille inspection, while GlobalVision completes in less than 4 minutes with 2X the accuracy.
- Eliminates the need for multiple systems, cutting costs and streamlining operations with all braille inspection features embedded within the robust GVD platform
- Ensures braille labeling compliance with Marburg Medium Font Standard and ISO for medical products, preventing regulatory issues
- Global scalability by inspecting braille translations in 44 languages
- Enhances braille inspections with detailed reporting on each individual braille dot across all regions, including multi-panel areas
Unmatched Braille Inspection Speed and Precision
With 10X faster braille height inspections and the support of full-size press sheets, GlobalVision’s newest automation technology surpasses the traditional DotScan software and all other competitors on the market. Printing & packaging and pharmaceutical companies can now expect to automate braille inspections with granularity and speed that was previously not possible.
The technology not only accelerates the inspection process but also ensures unparalleled precision, reducing the risk of oversights. Beyond heightened efficiency, the increased automation of the entire compliance inspection process allows organizations to redirect resources to more strategic tasks, fostering innovation and growth internally.
GlobalVision’s braille inspection technology redefines industry standards by combining speed and granularity, transforming compliance inspections into a faster, more comprehensive and accurate process.
Save Resources and Eliminate Unnecessary Overhead
With this new release, all braille inspection features are embedded within the robust GVD platform. This allows companies in regulatory industries to save money and resources by eliminating the need and overhead of managing multiple systems.
The GVD Braille Module consolidates all packaging quality control processes as it is fully embedded within the robust GVD system, allowing for text, graphics, barcode inspections and more, rendering the use of a separate system obsolete.
This upgrade ensures that every aspect of your quality control process can be seamlessly executed within a single platform. The efficiency gains are unmatched, as companies can now navigate the entire process, including the previously tedious braille inspection, with speed and precision, while simplifying their workflows and enhancing overall productivity.
This innovation not only streamlines operations but also positions the GVD Braille Module as an industry leader, offering unparalleled ease and efficiency to the market.
Ensure Adherence to Compliance Regulations
For regulated companies, the need to adhere to strict guidelines and requirements is critical. GlobalVision’s latest advancement in braille inspection technology represents a crucial upgrade in addressing this concern by guaranteeing ongoing braille compliance with the Marburg Medium Font Standard as well as the ISO 17351:2013 requirements for braille labeling on medicinal products.
This upgrade not only ensures precision and accuracy in braille, but, more importantly, serves as a proactive measure to prevent potential regulatory sanctions. By aligning seamlessly with established standards, this technology provides regulatory companies with the assurance that their braille labeling processes are not only efficient but also in strict accordance with mandated guidelines, protecting against potential legal and regulatory penalties.
Global Scalability
The GVD Braille Module isn’t confined to a specific language or region. It scales globally, inspecting braille translations in an impressive 44 languages against a master file. This global scalability ensures that the technology is not only cutting-edge but also inclusive and diverse, catering to the many linguistic needs of multinational enterprises.
By accommodating such a wide linguistic range, the GVD Braille Module becomes a versatile tool that addresses the diverse needs of companies worldwide, acting as a positive lever toward their global expansion strategies.
Detailed Reports and Compliance Data
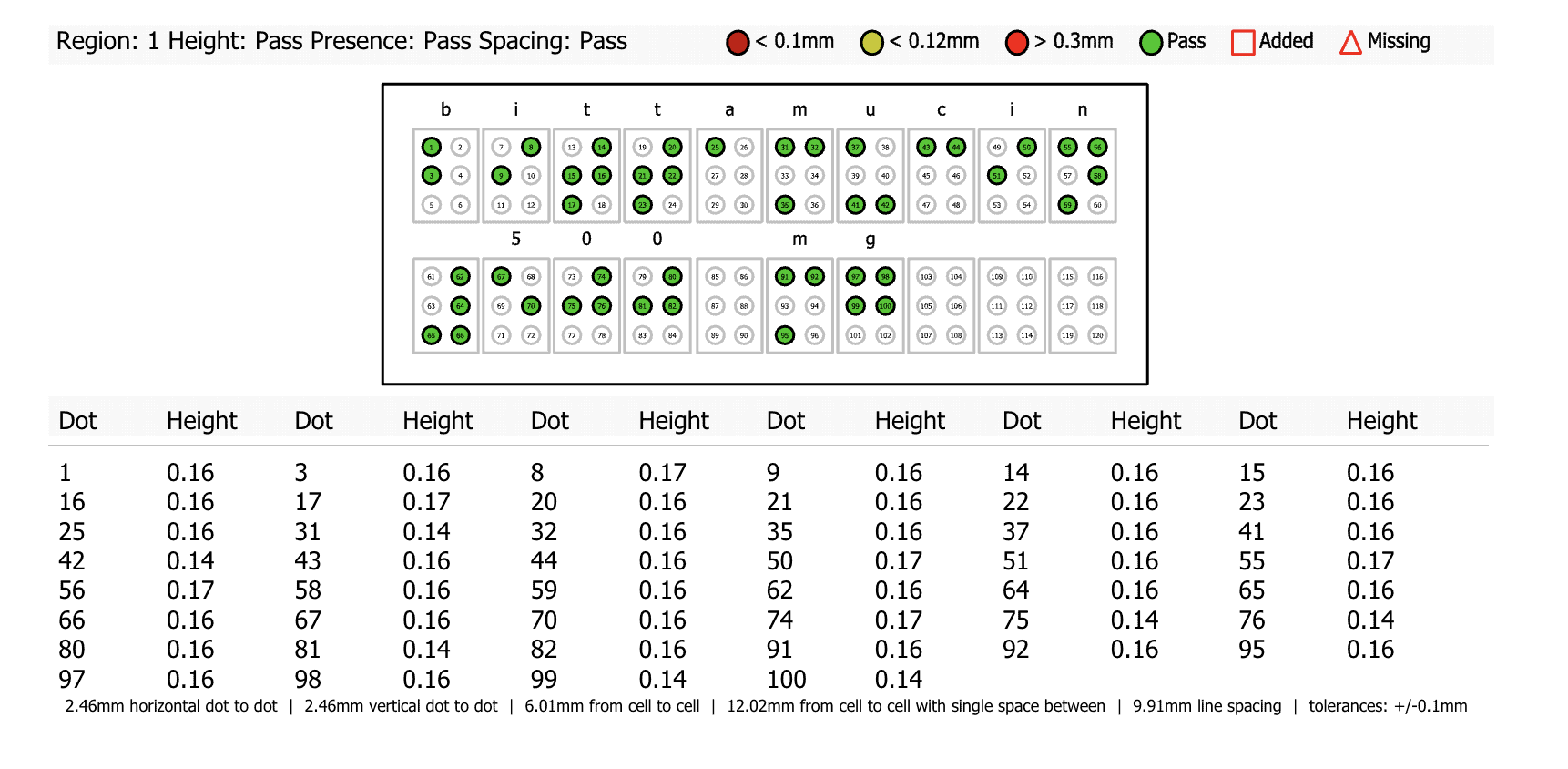
One of the standout features of the GVD Braille Module is its ability to provide you with highly detailed reports for each braille dot across all regions, ensuring that every single dot meets regulatory standards.
The GVD Braille Module allows you to achieve unprecedented granularity in braille inspections with in-depth reporting for each individual braille dot across all braille regions, including multi-panel regions. This means the software goes beyond basic inspection data and provides a level of inspection scrutiny unparalleled in today’s market.
The Future of Braille Inspection
In an era where technology and automation is the driving force behind progress, the GVD Braille Module stands out as a revolutionary solution in the field of braille inspection. This comprehensive solution surpasses all previous inspection systems and doesn’t just meet industry standards but sets a new benchmark for efficiency and accuracy.
As industries strive for greater innovation in technological solutions, the GVD Braille Module allows you to overcome all previous inspection limitations and embrace new technology that improves the entire quality control process.
GVD Braille Module is not just an upgrade, it’s a revolution in braille inspection. To try the new standard in braille inspection yourself, book a personalized braille demo and begin revolutionizing your compliance inspections today.