Date: April, 2024 | Category: Compliance | Author: Hana Trokic
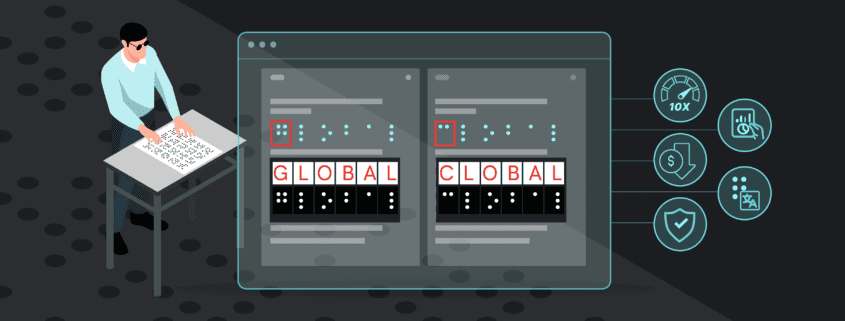
GlobalVision’s Braille Height Enterprise is breaking new ground by offering unmatched precision and speed never before seen for regulatory Braille inspections. With the new Braille Height Enterprise, inspections on pharmaceutical packaging can be completed in record time and with complete accuracy, getting products out to market faster than ever before.
This fully automated scanner-based solution is the first detailed Braille height measurement solution that measures the exact height of each individual Braille dot. Previously, teams had to rely on manual inspection methods or had to suffice with automated solutions giving an average measurement value across Braille regions instead of exact measurements, resulting in compromised compliance in the EU market.
GVD Braille Height Enterprise not only eliminates the need for these error-prone and time-consuming manual Braille inspections, it also sets a new standard for speed of automated Braille inspections for professionals in regulated industries including pharmaceuticals and print and packaging.
The GVD Braille Enterprise inspection software is a one-of-a-kind solution on the market allowing teams in quality assurance and prepress to confidently meet regulatory standards and ensure accurate Braille assets leading to safer and more accessible products, marking a significant step forward in technology-driven quality assurance.
The Importance of Braille Accuracy
In regulated industries, where adherence to standards and accessibility is crucial, Braille is a vital component of a product’s packaging or labeling to foster compliance and inclusivity.
Braille, a universally accepted system of writing designed for individuals with visual impairments, is critical in ensuring that information, particularly in industries subject to strict regulations such as pharmaceuticals, is universally accessible.
The importance of Braille accuracy on pharmaceutical packaging and labeling cannot be overstated as it directly impacts the health and safety of consumers and patients with visual impairments. Taking into consideration that there are currently 295 million people worldwide with moderate to severe visual impairment, the need for Braille only becomes more pronounced.
In pharmaceuticals, where a single mistake can lead to serious health complications or even life-threatening consequences, the accuracy of Braille is a vital component of patient safety. Accurate Braille, including height, spacing, and translations, ensures that patients can independently access critical information regarding dosages, ingredients, and usage instructions, significantly reducing the risk of misuse.
Yet, ensuring the accuracy of Braille assets is not just a matter of health safety, it is also a question of legal compliance and ethical responsibility.
From a quality assurance and production perspective, Braille accuracy is vital as it is a mandatory component on pharmaceutical packaging all across Europe, governed by the European Medicines Agency (EMA) and is strongly recommended by the U.S. Food and Drug Administration (FDA) in Western markets, while other regions such as the ASEAN markets are working to heighten inclusivity by implementing Braille requirements on packaging soon.
Get To Market Faster Than Before
By harnessing automated technology, the GVD Braille Height Enterprise is 10X faster than competitor solutions and cuts down inspection times dramatically, from what would previously take several hours, down to just a few minutes. This drastic reduction in inspections enables teams to handle larger volumes of inspections with the same (or less) labor resources, boosting productivity and streamlining operations.
In the competitive EU medicines environment, time to market is a critical factor for success. The Braille Height Enterprise inspection software streamlines inspection processes for Braille assets, allowing products to move more quickly from production to market. This speed can provide a competitive advantage, enabling companies to capture market share more effectively. It also aids teams specifically in pharma packaging to produce fast and accurate products allowing for life saving medicines and drugs to reach consumers more quickly.
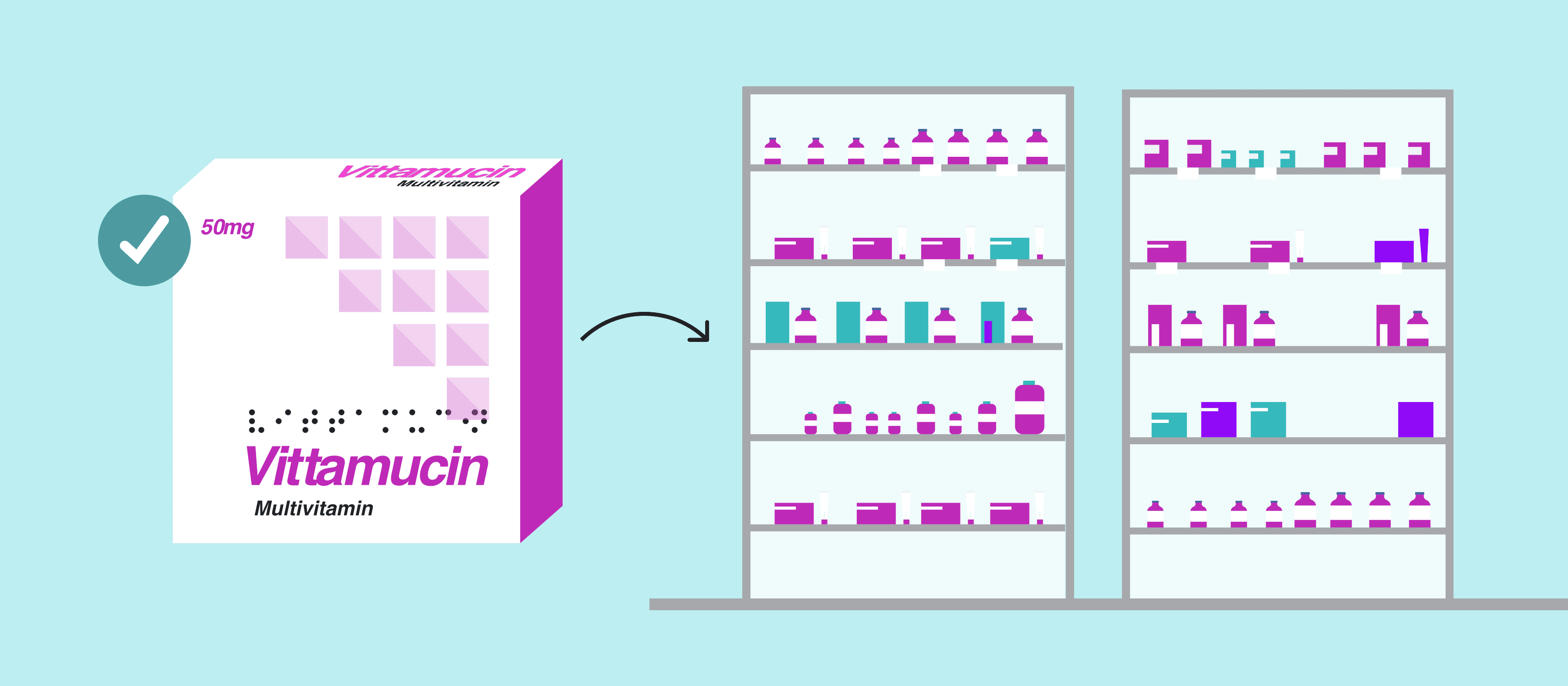
In under 3 minutes, the software can accurately inspect Braille height for each individual dot, and all other aspects of Braille across various materials and formats, including foil cartons and full press sheets. It can also inspect and translate Braille in 43 languages – the highest amount of supported languages available for Braille translations – allowing for easier global scalability.
These metrics once again set GVD Braille Height Enterprise apart from other solutions as its automated technology is more robust and comprehensive than any other solutions on the market.
For perspective, competitor software on average takes 50 mins to complete a full Press sheet Braille inspection, while GVD completes the same inspection in less than 3 minutes. What’s more, inspections can be done in 43 global languages, on different materials, across multiple Braille regions, and can be conducted simultaneously, with other GVD capabilities such as graphics, text, color, and barcode inspections.
All of these capabilities allow teams to accelerate their inspection times and get vital pharmaceutical products to markets all across Europe at never before seen speeds.
Experience Unmatched Braille Accuracy Through Precise Inspections
Traditionally, Braille inspections have been conducted manually to ensure the accuracy of Braille on packaging, labeling, and other regulatory documentation. This method involved trained personnel visually inspecting for obvious defects such as missing dots, misalignment, or inconsistencies in the Braille pattern. It also involved tactile inspections where Braille would be manually felt to ensure that the dots are raised to the correct height, are distinguishable to the touch, and are translated properly.
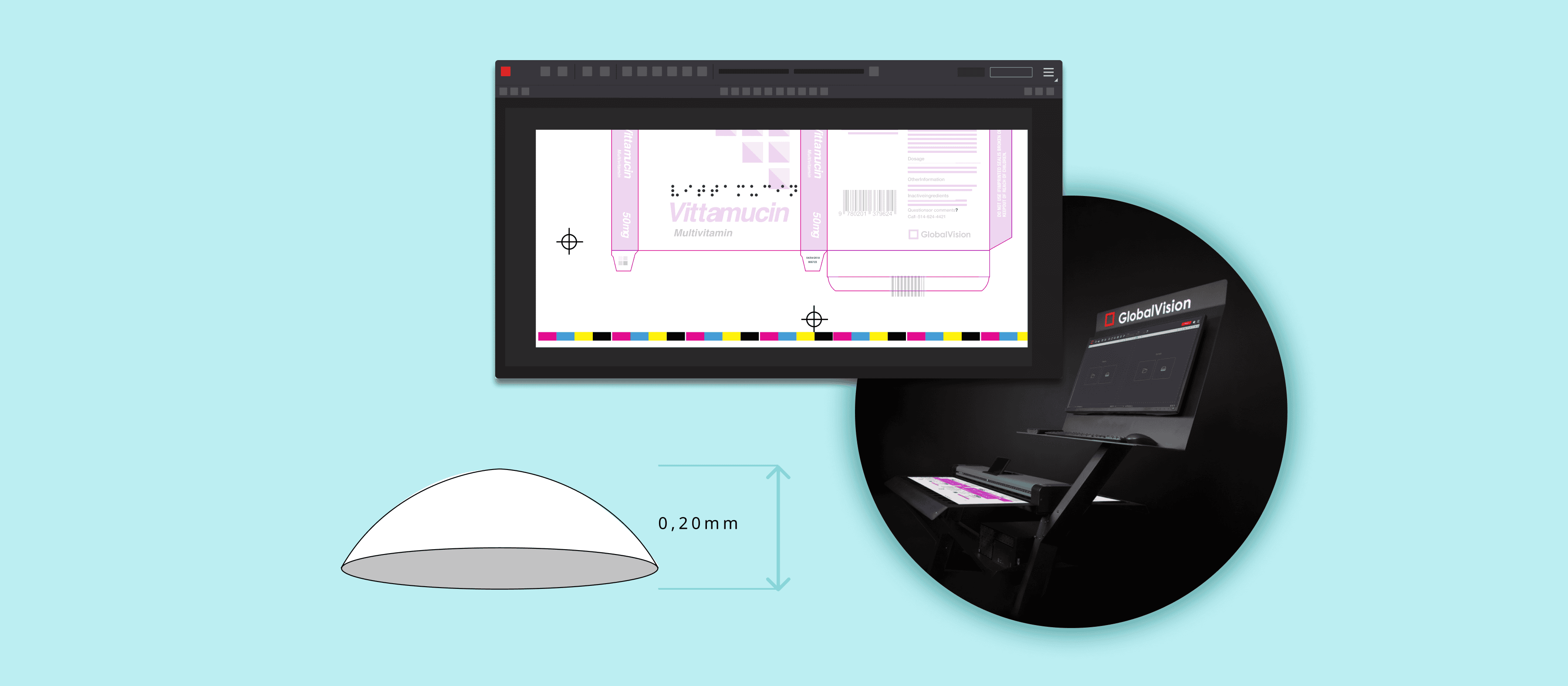
Due to the high risks that come with manual inspections, these methods have proven to be unreliable verification processes that are highly susceptible to human error, including oversight and fatigue. The GVD Braille Height Enterprise mitigates these risks by automating the inspection process, thus significantly lowering the chances of mistakes that can compromise the quality and compliance of Braille outputs.
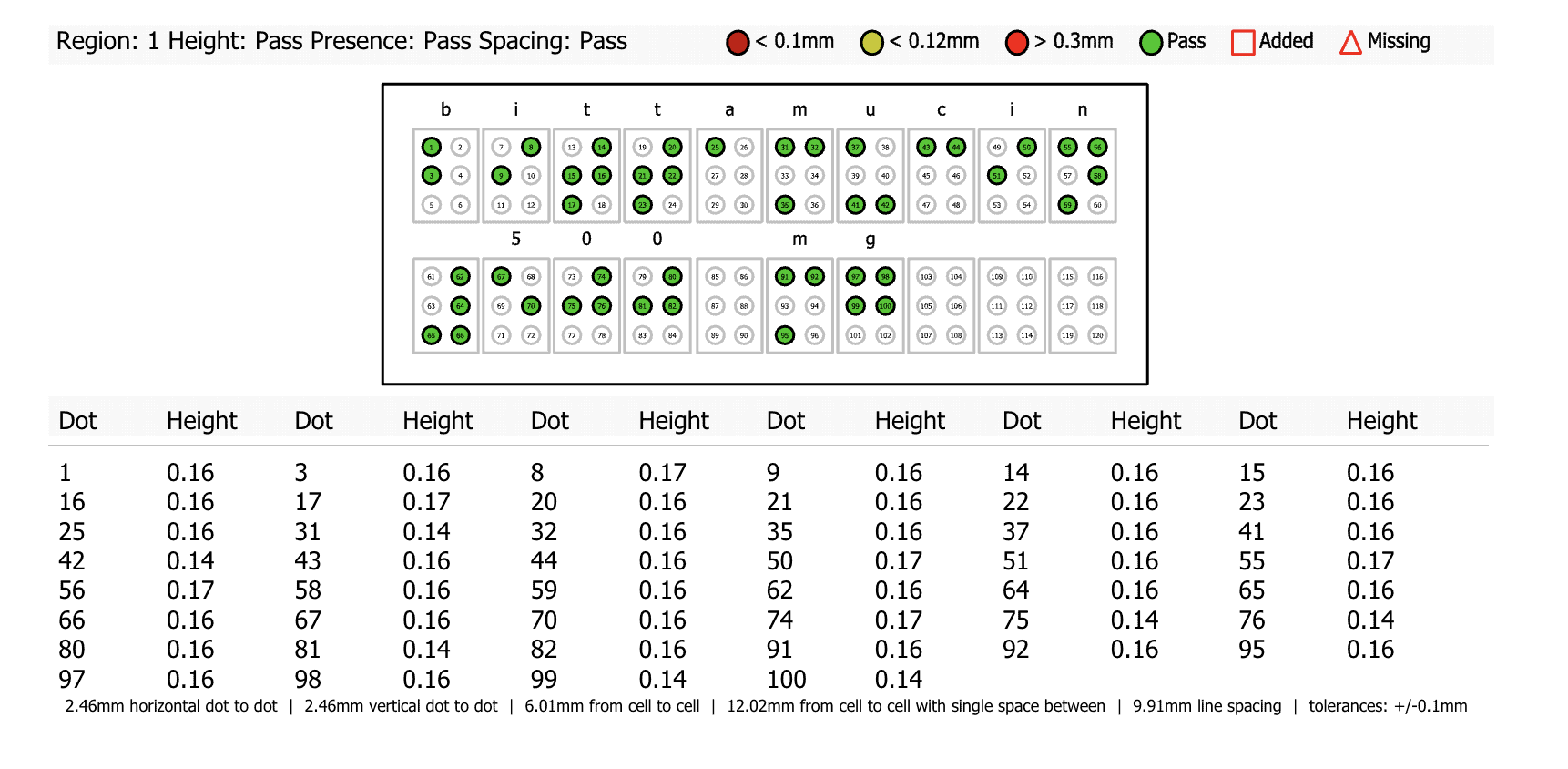
The Braille Height Enterprise inspection software enables teams to check for Braille accuracy through automated inspections that check for Braille height, spacing, translations, missing dots, and more in one comprehensive scan. For Braille height specifically, the software inspects the exact height of each individual Braille dot ensuring the accuracy of Braille assets with extreme precision.
In a matter of minutes, the inspection will yield a detailed report of all non-compliant Braille dots on packaging assets, allowing teams to make adjustments accordingly and ensure the complete accuracy of their regulatory documentation.
Currently, GVD Braille Height Enterprise is the most accurate on the market, yielding inspections that are 2 X more accurate than competitor solutions.
Have Complete Confidence in Your Compliance
For pharmaceutical and packaging industries, the accuracy of Braille on packaging and labeling is critical. This is due to the fact that errors in Braille can lead to devastating non-compliance issues.
When it comes to inspecting Braille accuracy on regulatory content such as packaging and labeling files, the process is done to ensure, amongst many things, the compliance with the Marburg Medium Font Standard as well as ISO 17351:2013 requirements for Braille on pharmaceutical products.
Braille inspections in particular have posed challenges to the pharmaceutical and print and packaging industries as stringent regulations such as the Marburg Medium Font Standard requires a height of 0.20mm, with many companies producing at a height of 0.10mm. This makes Braille’s height incredibly difficult to measure through manual inspections and heightens the risk of errors slipping through that can lead to non-compliance issues.
GVD Braille Height Enterprise addresses these unreliable and inefficient manual verification processes, reducing risks of incorrect Braille translations, tactile issues, spacing errors, and spelling mistakes, all of which cause compliance issues such as financial penalties, recalls, risk to consumer and patient safety and damage to a company’s reputation.
This automated solution detects even minor discrepancies in Braille assets by inspecting each individual Braille dot, thereby increasing the accuracy and reliability of Braille asset verification. Not only does it render manual inspections obsolete, it outpaces other automated solutions that only give average measurement values across Braille regions.
This advanced technology allows teams and organizations to avoid the consequences of non-compliance as they can now meet regulatory standards with greater precision, accuracy, and speed.
A New Standard for Braille Inspections
Due to its never-before seen accuracy, precision, and speed, the GVD Braille Height Enterprise is a market-leading solution that sets a new standard for Braille inspections.
Incoming quality assurance and production teams in the pharmaceutical and print and packaging industries can now leverage the latest and most innovative Braille inspection technology to eliminate bottlenecks and get critical products out to market all across the European Union faster than any other competitor.
By replacing slow, inefficient, error-prone manual inspections with fast, accurate, and automated technology, the Braille Enterprise inspection software eliminates risks associated with human errors in Braille verification which include incorrect translations, tactile issues, spacing errors, and spelling mistakes all which can all lead to compliance issues, financial penalties, recalls, risks to consumer and patient safety, and damage to a company’s reputation.
If you want to discover the full capabilities of our innovative GVD Braille Height Enterprise, book a demo today and see firsthand the transformative impact it will have on the accuracy, speed, and compliance of your regulated Braille assets.



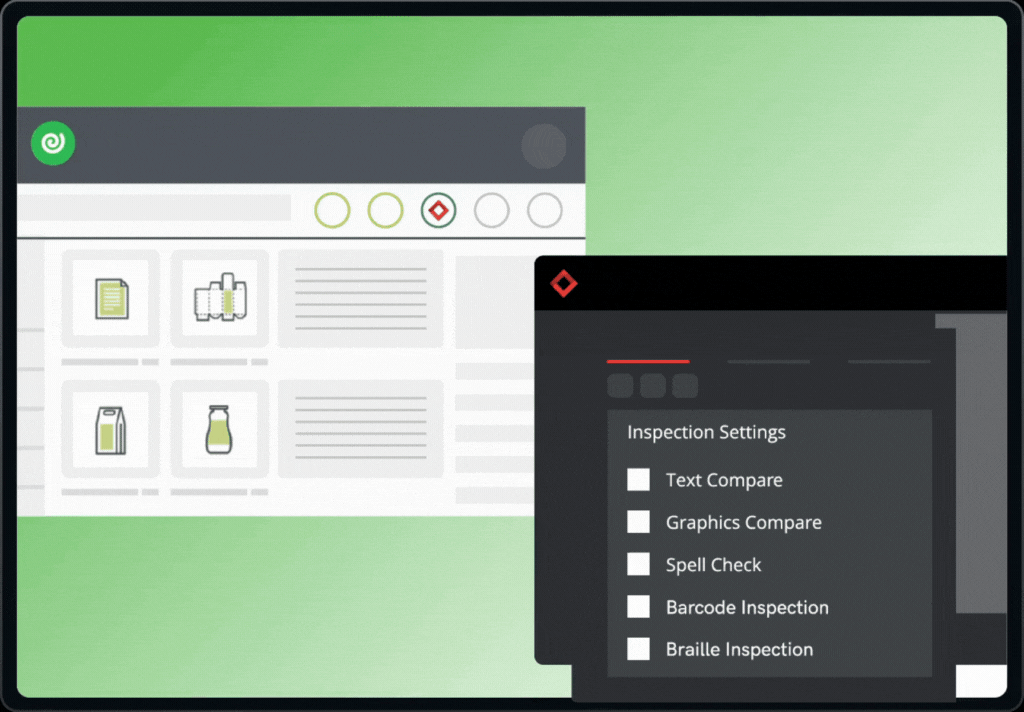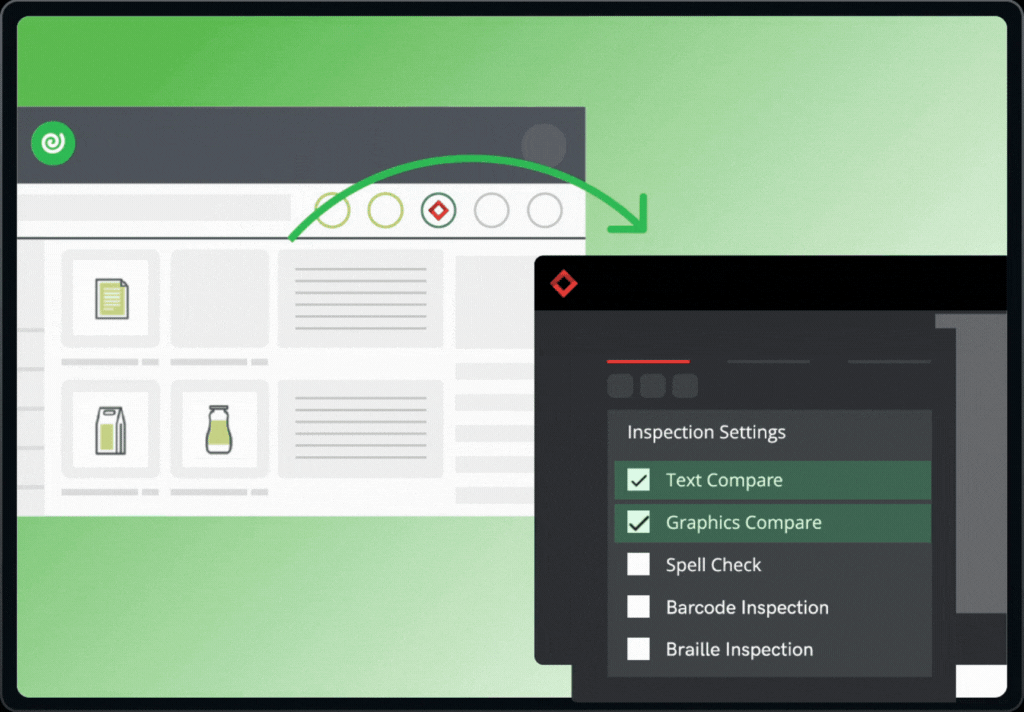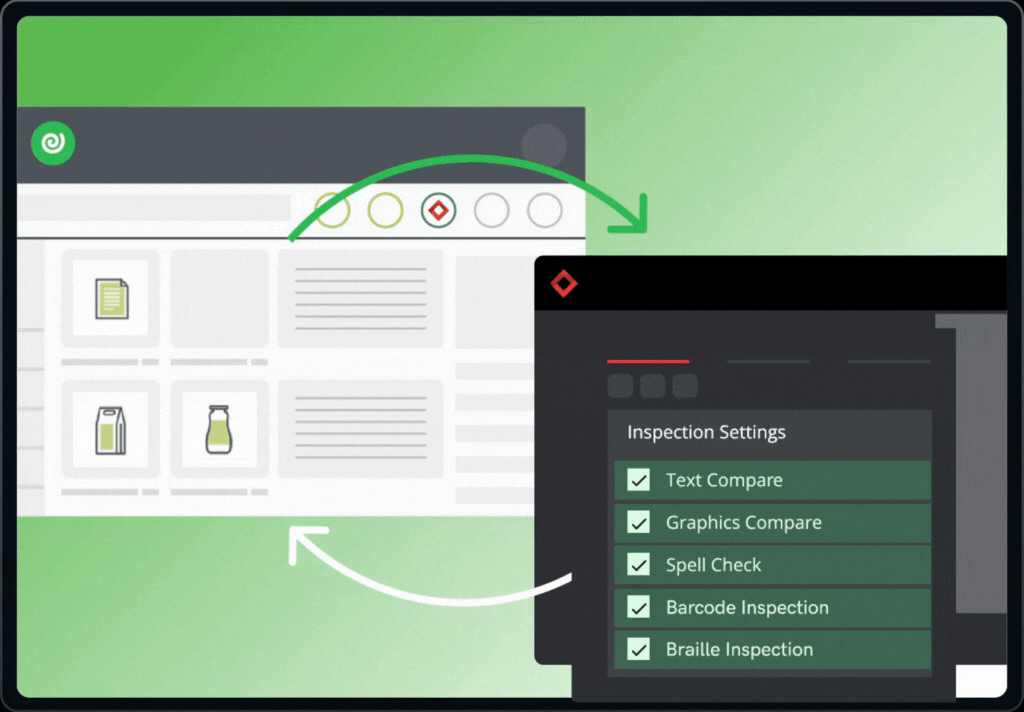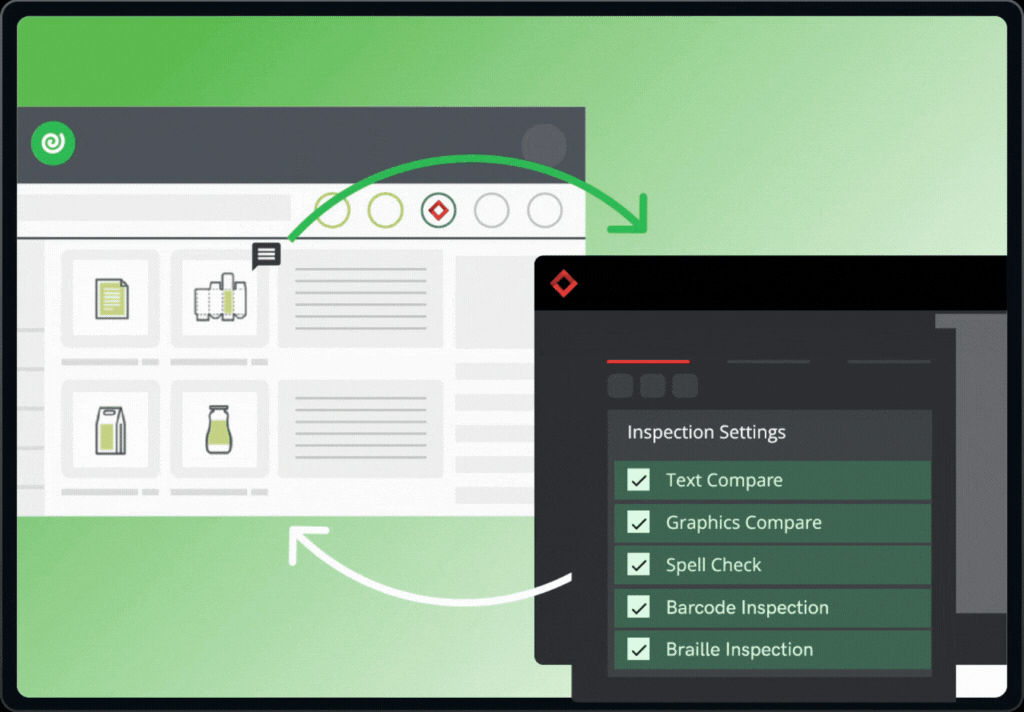 The integration brings together
The integration brings together