Date: August, 2023 | Category: Customers | Author: Hana Trokic
About IPG Health
IPG Health is a network of world-renowned agencies focused on health communication and marketing. Collectively, the network is made up of over 45 agencies that are spread across six continents and 6,500+ employees driven by a company-wide obsession with harnessing creativity, technology, science, and data to inspire behaviors that fuel better health.
IPG Health’s clients include the top 20 global pharmaceutical companies as well as countless startups, biotech companies, biopharma companies, and a variety of life science companies. In everything they do, IPG Health is relentlessly focused on doing what’s right for their clients, their brands, and their people.
The Challenge: Keeping Content Accurate While Facing Regulatory and Time Constraints
Due to the fast-paced, highly regulated, and critical nature of their field of work, IPG Health must focus significant time and resources on ensuring the accuracy of every piece of content and creative material produced.
Kyle Richards, EVP, Executive Director, Editorial, at IPG Health, gave us a detailed look into some of the key challenges his teams and department face in the project development process. He noted that their main goal was to deliver accurate content for their clients and a large part of ensuring that was through accurate and fast proofreading processes.
IPG Health deals with large volumes of medical and scientific materials that are often lengthy and technical, and that include special characters that further complicate proofreading. When reviewing the documents manually, the proofreading process can be very time-consuming. Traditionally, this often led to the need for additional proofreaders at the late stages of the project development process to avoid bottlenecks while ensuring accuracy.
Further, duplicate proofreading reviews at key project milestones were part of their standard operating procedure to ensure accuracy as projects moved through the late-stage production steps. The need for a second proofreader so routinely added a resource management challenge to the quality control process.
“We found that there were bottlenecks with regard to turning projects around and getting them back to our clients. We were coming up against these crunch times to turn around content quickly for product launches, and the manual proofreading process was slowing us down.”
- Kyle Richards, EVP, Executive Director, Editorial, at IPG Health
The Solution: Increase Content Review Efficiency and Maintain Accuracy Through Automation
When IPG Health first sampled GlobalVision’s automated solutions 10 years ago, they instantly noticed the incredibly accurate results it was producing proofreading files. On top of that, even at a time before the routine use of digital tools, GlobalVision products were easy enough to use to enable a broad rollout within the editorial teams.
For over a decade, this accuracy has proven reliable, as IPG Health still trusts GlobalVision as an important component of its editorial process.
“A lightbulb went off when we were proofreading a 35-page product package label. The manual proofreading took most of the day to complete, yet when I ran the files through [GlobalVision], it took all of 30 minutes. It caught all the errors the manual proofreader found plus two additional errors that would have otherwise slipped through.”
- Kyle Richards, EVP, Executive Director, Editorial, at IPG Health
In many cases, IPG Health teams work with pre-approved client content that needs to be included in their materials. While there is plenty of room for their renowned creativity in other areas, in these cases they must strictly adhere to the approved language. To help with this, GlobalVision is used to ensure complete accuracy, in addition to its use for late-stage proofreading to ensure content integrity.
Kyle Richards notes that due to their text-heavy content, the Text Inspection tool is the feature they rely on most. They also benefit greatly from the built-in Spell Check and Custom Dictionaries, which proved to be extremely helpful when proofreading health-related material. Graphics inspection is also used frequently, as diagrams, charts, and visuals are commonly included in the content.
Throughout the years, IPG Health found numerous advantages of using GlobalVision’s file comparison technology but has particularly noted three main software benefits:
- Accuracy: Maintains accuracy of proofread documents and provides an additional layer of quality control in late-stage reviews.
- Time Savings: The addition of automated inspections drastically decreases the time needed to proofread lengthy, technical, health-related documents, particularly in late-stage reviews.
- Better Use of Expertise: Provides an added layer of support and verification for editors and allows them more time to perform other high-value aspects of their role.
Automated inspections drastically decrease the time needed to proofread lengthy, technical, health-related content. In general, it decreases proofreading times at IPG Health by about 75%-80%.
It hasn’t been just IPG Health reaping the benefits of GlobalVision, their clients have also received the benefits. Kyle Richards notes that “Clients are always looking to be more efficient with their budget. With GlobalVision, we’re able to do more work within the same time, meaning they’re getting more for their dollar.”
“Clients are always looking to be more efficient with their budget. With GlobalVision, we’re able to do more work within the same time, meaning they’re getting more for their dollar.”
- Kyle Richards, EVP, Executive Director, Editorial, at IPG Health
As they are continually working to improve the efficiency of their quality control processes, IPG Health recently began implementing GlobalVision’s newest innovation in cloud-based proofreading software, Verify. During the switch to the latest software, the editorial teams noted that it was very easy and intuitive to use and that it has made proofreading even easier than before.
For a decade and counting, IPG Health has had nothing but praise for GlobalVision and the advantages it has added to its quality control workflow. When asked if he would recommend the software to others considering automated proofreading, Kyle Richards quickly answered, “Yes, absolutely. From an editorial perspective, it supports our proofreading efforts and allows us to do a challenging aspect of our role much more efficiently, which also buys us more time to tackle other aspects of our role that bring higher value to the team.”
Take the first step toward error-free content with GlobalVision’s automated solutions. Learn how proofreading software can help your quality control workflow, and start reaping the benefits today.
 Biogen is a leading multinational biotechnology company specializing in therapeutics and medicines for the treatment of neurological diseases, specialized immunology, and rare diseases. Pioneering innovative science to deliver new medicines that transform patient lives, Biogen operates internationally with offices across the Americas, Europe, and Asia.
Biogen is a leading multinational biotechnology company specializing in therapeutics and medicines for the treatment of neurological diseases, specialized immunology, and rare diseases. Pioneering innovative science to deliver new medicines that transform patient lives, Biogen operates internationally with offices across the Americas, Europe, and Asia.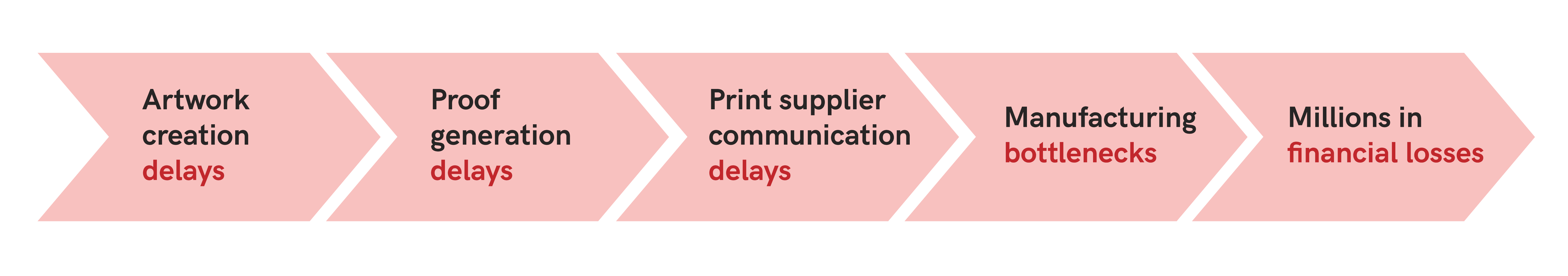 Solution: Streamline Artwork Development With Automated Quality Inspections
Solution: Streamline Artwork Development With Automated Quality Inspections ![]()