Date: January, 2024 | Category: Company | Author: Hana Trokic
Introducing our Verify Winter Release.
Our latest version streamlines the addition of new terms with a new approval workflow for Custom Dictionary words, accelerating your content review process while reducing unwanted spell check errors.
This exciting release also brings new features and improvements tailored to diverse sectors including pharma, CPG, print and packaging, marketing agencies, and more—promising to improve content compliance while optimizing your workflow.
Enjoy the following upgraded features of our Winter Release:
- An innovative approval workflow for Custom Dictionary words to simplify your process of adding new terms.
- A refined and optimized Optical Character Recognition (OCR) to save time and reduce non-compliance issues.
- A revamped Prep Stage and Filtering Tool in the Results Stage to go through differences more efficiently.
- The ability to manage Spot Colors in the Prep Stage—broadening your graphics inspection capabilities.
- UX improvements, including the graphics inspection functionality now capture Live Text Differences in more easily identifiable red boxes.
- Asian and Right-to-Left Language Text Inspection accuracy improvements to help scale your proofreading operations globally.
- A significant reduction in unwanted Spell Check Results for more accurate inspection results.
With the addition of these cutting-edge features, Verify’s Winter Release not only sets new benchmarks for precision, efficiency, and compliance but also pioneers innovative capabilities, opening new possibilities in automated proofreading for regulated industries.
If you want to learn more about how to leverage our new features, book a Verify demo and begin experiencing these innovations in proofreading technology.
Add New Words to Custom Dictionaries With Increased Ease
Verify’s Winter Release also introduces a new built-in Approval Workflow for Custom Dictionary Words.
Pharmaceuticals, CPGs, and marketing agencies deal with content that includes many customs terms that are not readily available in regular dictionaries. These words include drug and brand names, or certain ingredient names and terms that are not standardized. In order for these words to not be flagged as errors during inspections, Verify allows for the addition of terms into custom dictionaries to further streamline inspection processes.
Our new approval workflow for Custom Dictionary Words allows users to seamlessly request approval from administrators to add words to custom dictionaries, ultimately streamlining day-to-day spell check and proofreading operations.
Watch our video to see the Admin Custom Dictionaries feature in action
Watch our video to see the User Custom Dictionaries feature in action
OCR: Save Time and Reduce the Risk of Non-Compliance
Verify continues to offer the most cutting-edge technology in cloud-based proofreading software by refining and optimizing our Optical Character Recognition capabilities. This feature allows users to inspect flattened text on documents such as labels, promotional material screenshots and supplier proofs by converting the digital images into readable, live text format.
Verify’s OCR feature leverages powerful artificial intelligence technology that detects characters within images or flattened documents, rendering a precise character-for-character text inspection possible.
This feature helps you save time on inspections and reduce the risk of non-compliance errors falling through. By automating the process of recognizing and extracting text from images or scanned documents, users can expect to drastically decrease the need for manual inspections, as well as the overall time needed to digitally inspect files.
By reducing manual processes, OCR also decreases the risk of human errors, safeguarding sensitive and critical documentation from errors that can lead to financial losses and the potential of a negative brand image.
While OCR is intended for all users, it will prove to be particularly beneficial for content, creative, regulatory, and labeling specialists in a variety of sectors from pharmaceuticals, print and packaging, prepress, and marketing agencies.
Watch our video to see the OCR feature in action
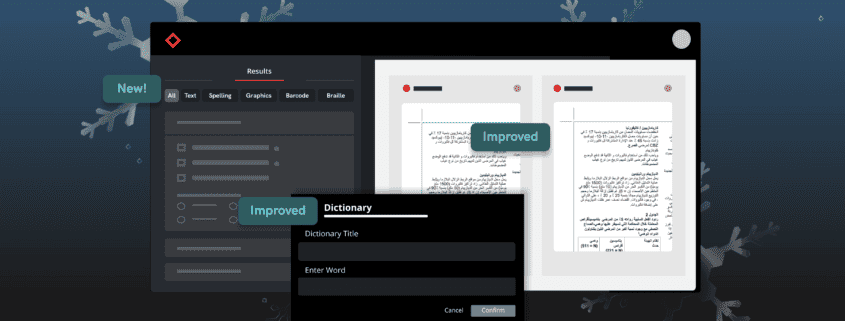
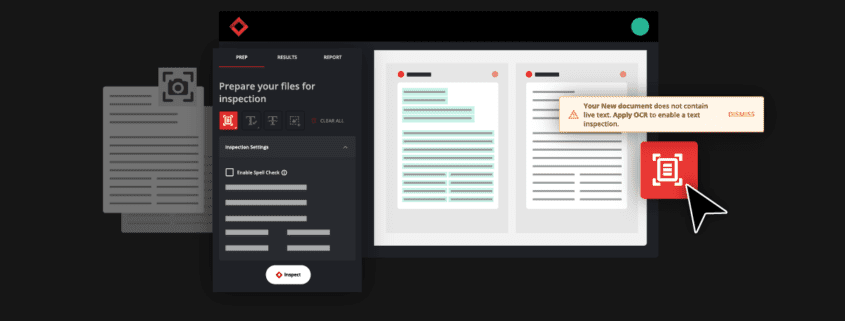
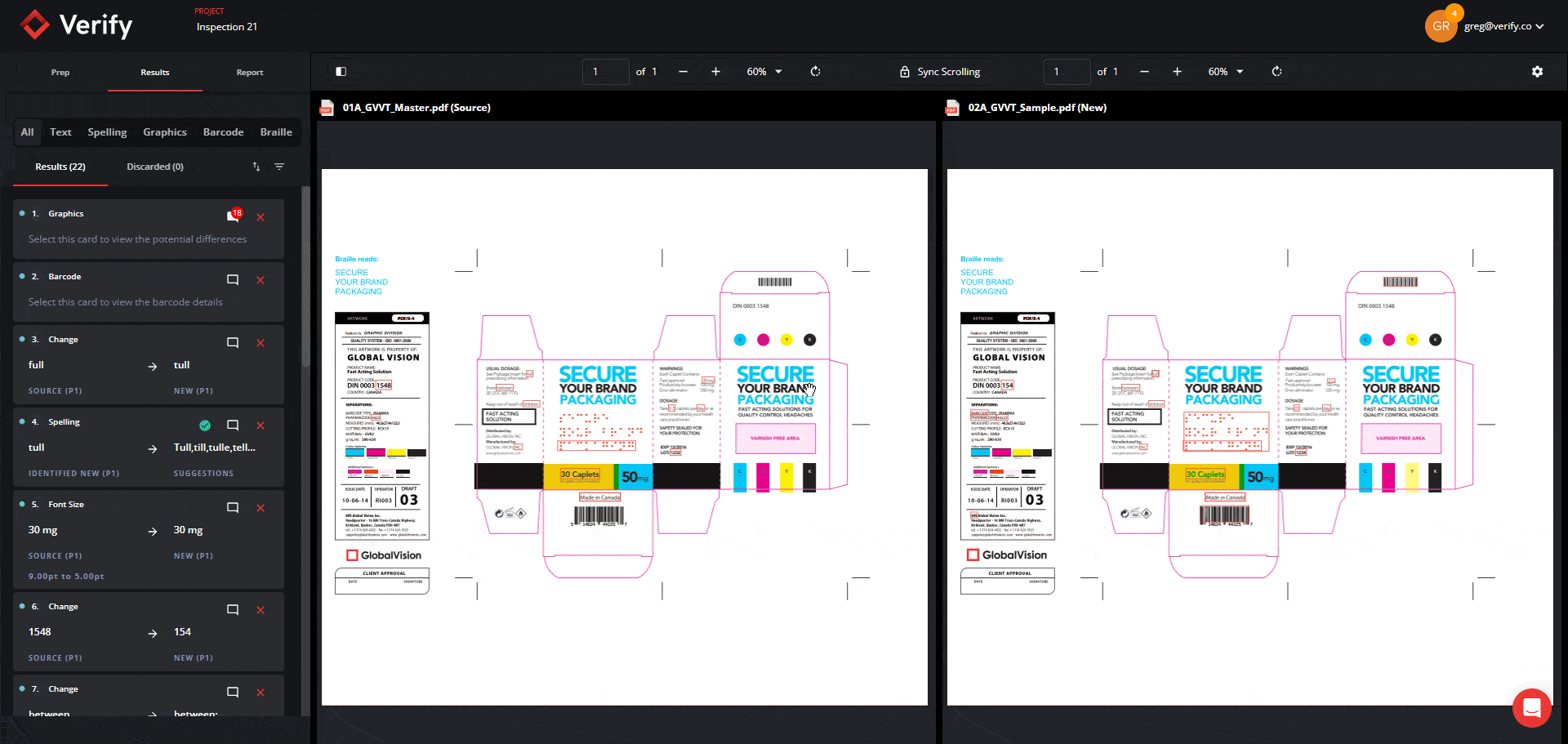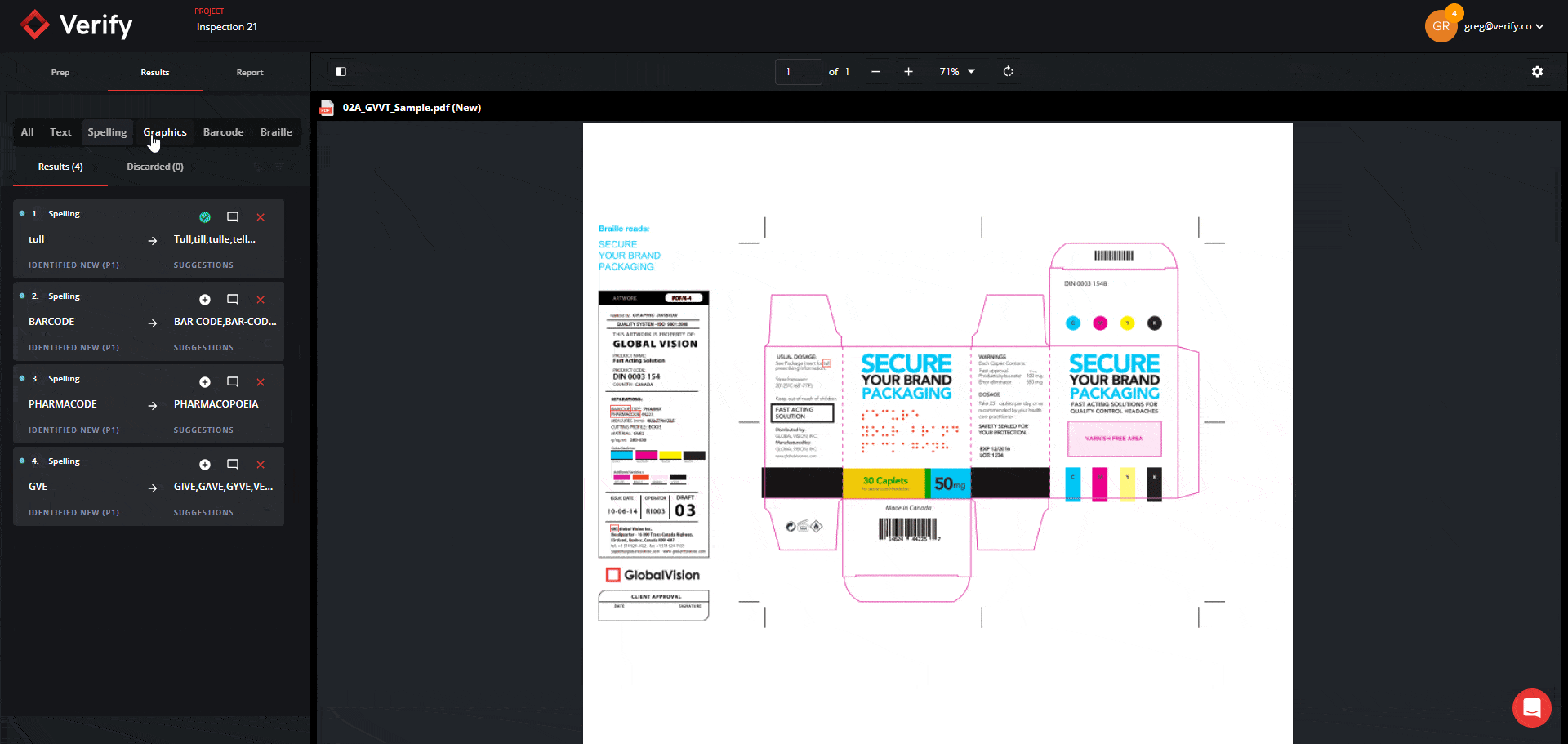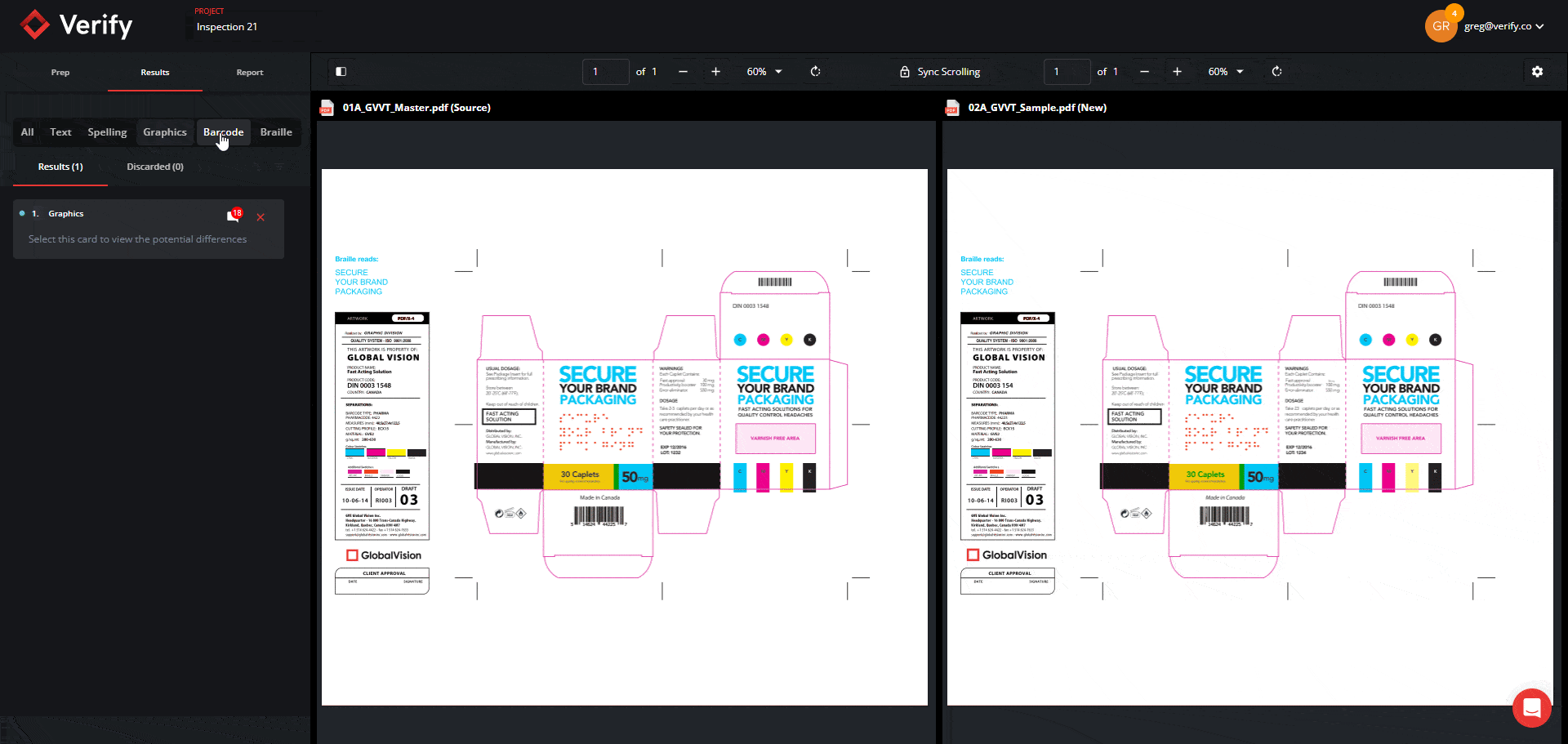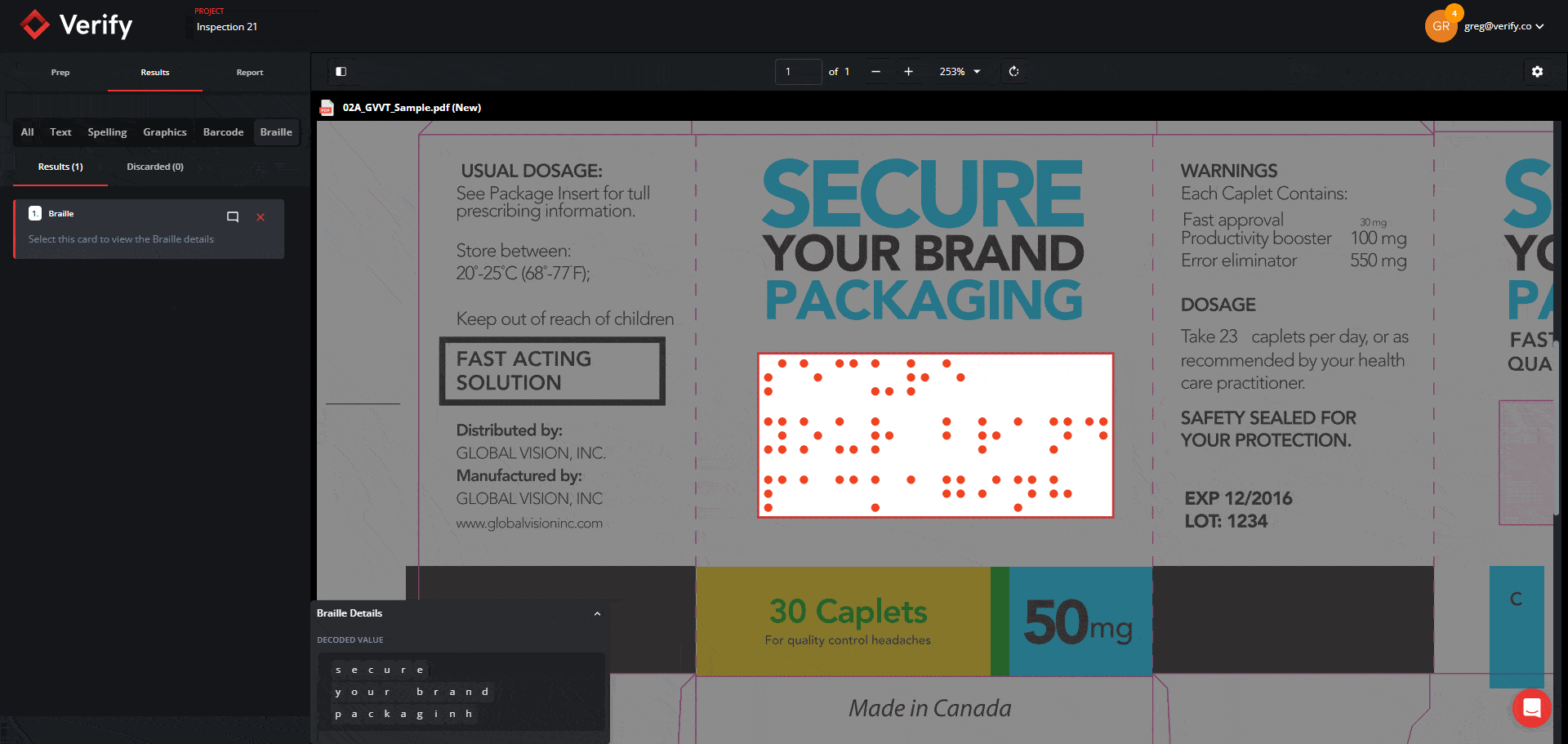
Revamped Prep Stage and Filtering Tool in the Results Stage
You can now expect to go through inspection results in record time through the new Prep Stage options and Filtering Tool in the Results Stage. Previously, users did not have the option to opt out of Text Inspection, and when reviewing inspection results, users sometimes had to go through large amounts of results cards with no clear and efficient method to filter and resolve them.
Now, users can select exactly which types of inspections they want to perform from the Prep Stage, and all Results get automatically segmented into 5 separate panels with their own unique default filters: Text, Spell, Graphics, Barcode, and Braille. You will also still have the option to view all results together with the “All” filter function.
This will allow you to view results in a more clear and concise manner and tackle differences by priority and importance, ultimately improving the revision process and allowing you to make corrections and improvements with greater ease.
See the new revamped Prep Stage and Filtering Tool below
Enhanced Graphics Inspection Capabilities
For those who heavily focus on graphics inspections, the Verify Winter Release also offers game-changing features to ensure the accuracy of your graphics.
Ability to Manage Spot Colors in the Prep Stage
This release also brings the ability to manage Spot Colors in the Prep Stage (also known as “Separations Control), expanding graphics inspection capabilities by ensuring a more comprehensive assessment of files, resulting in the heightened accuracy to your graphics files.
In the Prep Stage, a new option called ‘Spot Color Controls’ will now allow you to turn Spot Colors on or off to ease your revision process and allow you to view results and make needed adjustments efficiently.
Regulated industries such as pharmaceuticals, print and packaging, CPG, as well as agencies deal with complex workflows and often have limited power over the composition (layers and spot colors) of the files that need to be inspected. If the presence of a Spot Colors is hindering a successful graphics inspection from happening, the inability to turn that Spot Color off may reduce the accuracy of any given inspection, or make the inspection impossible. With the addition of this new feature, this bottleneck will be eliminated allowing for more seamless inspections.
Watch our video to see the Spot Colors Control feature in action
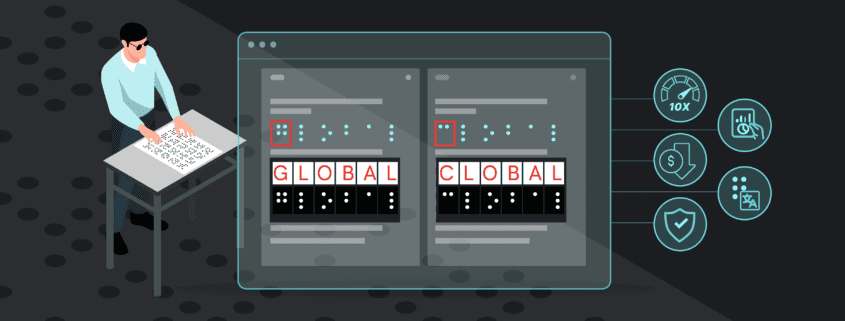
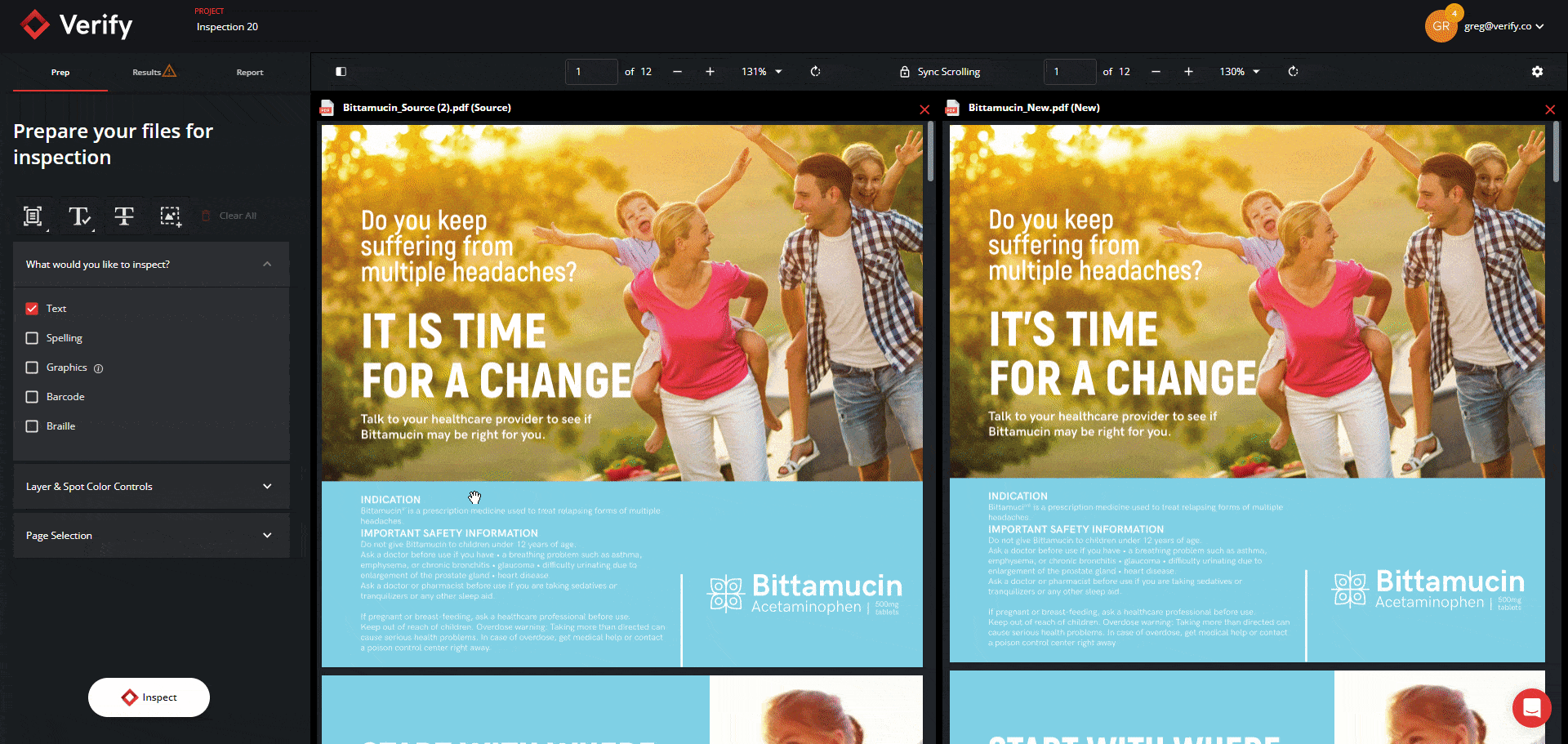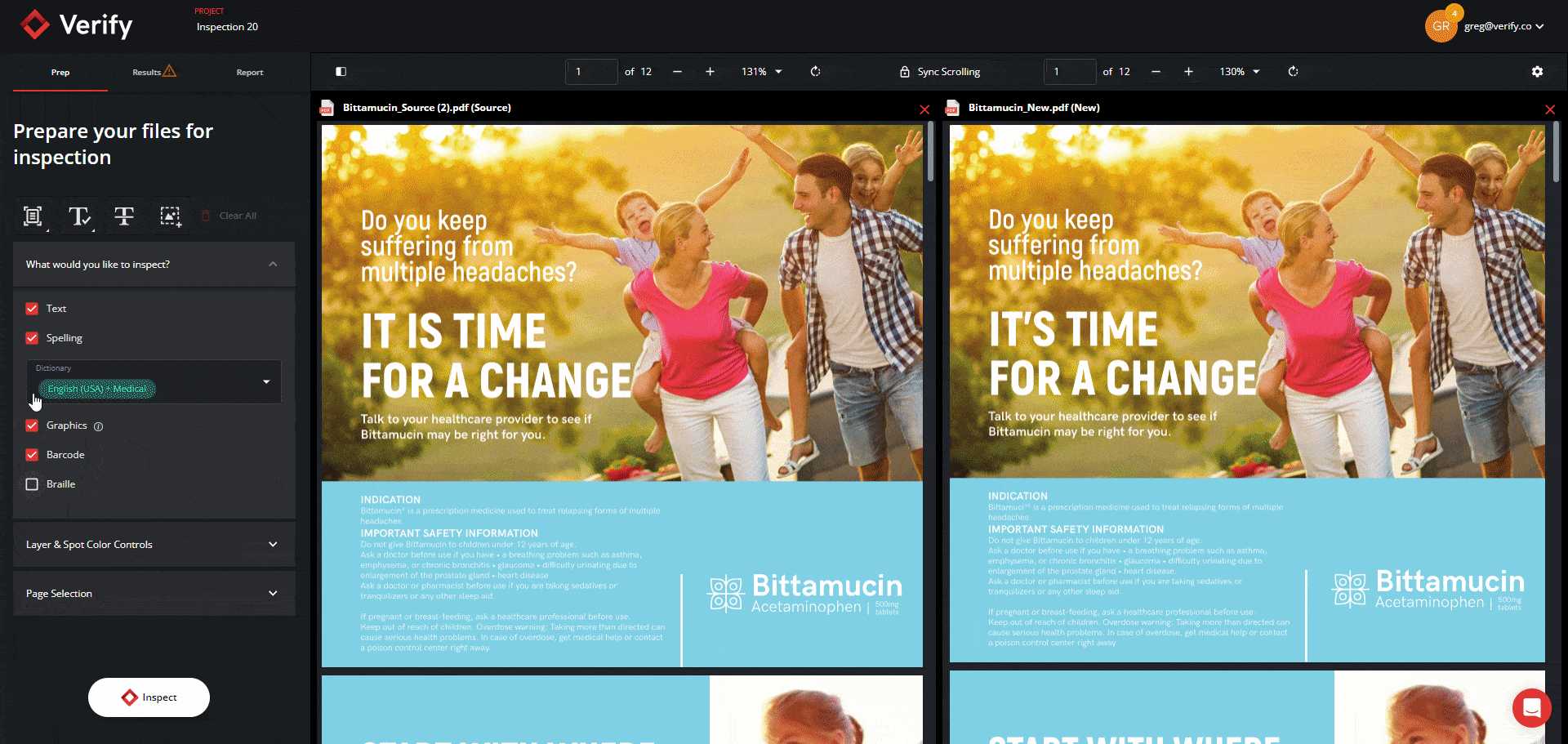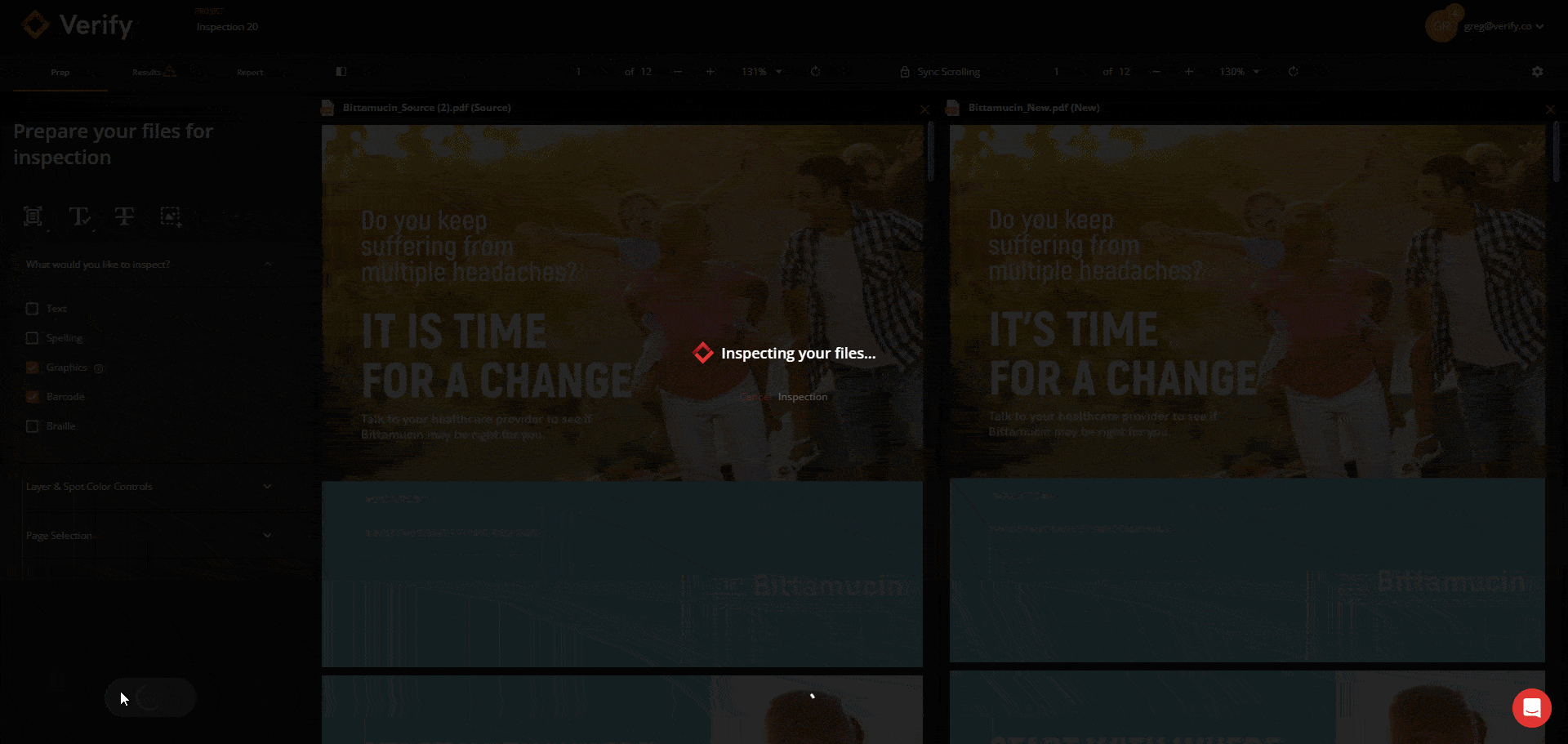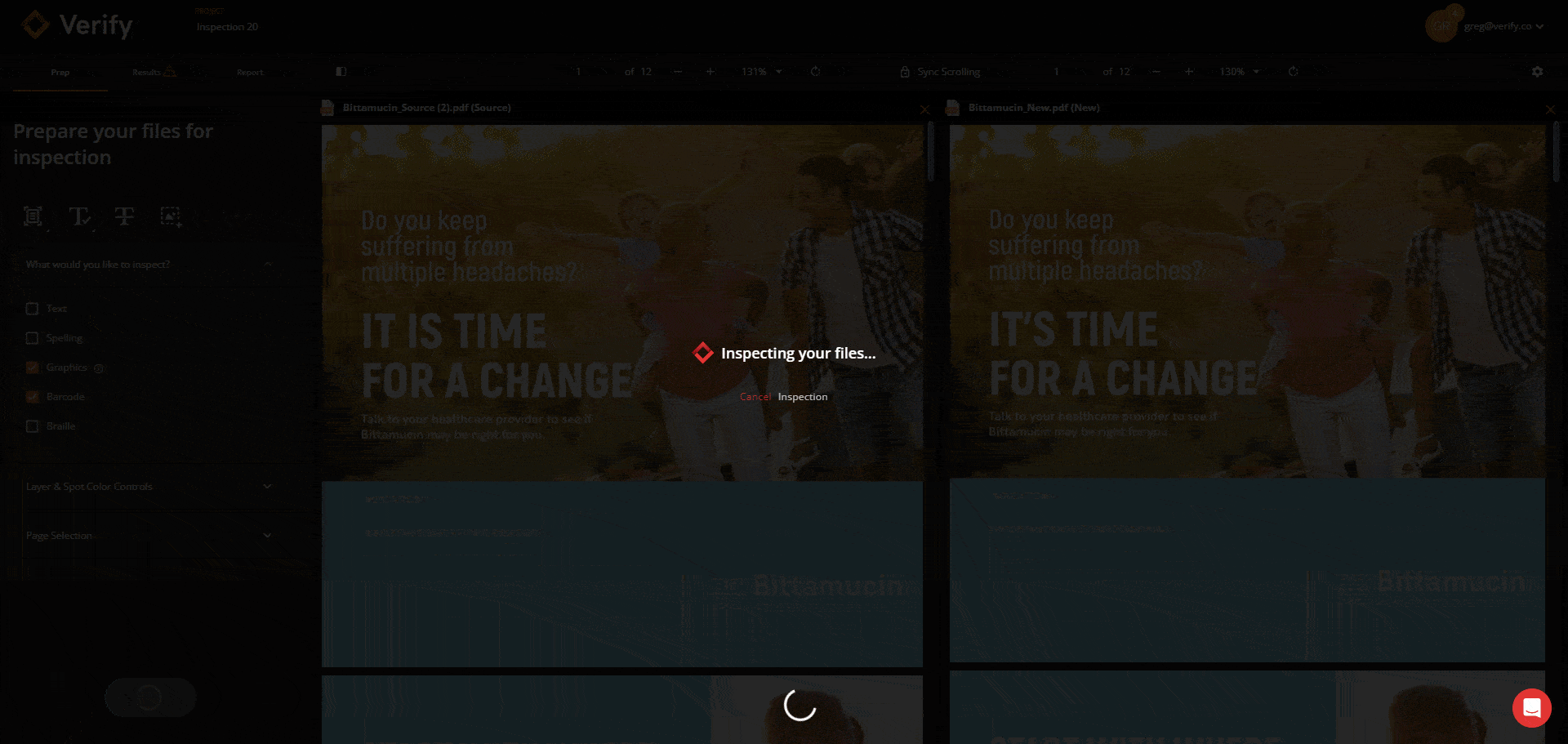
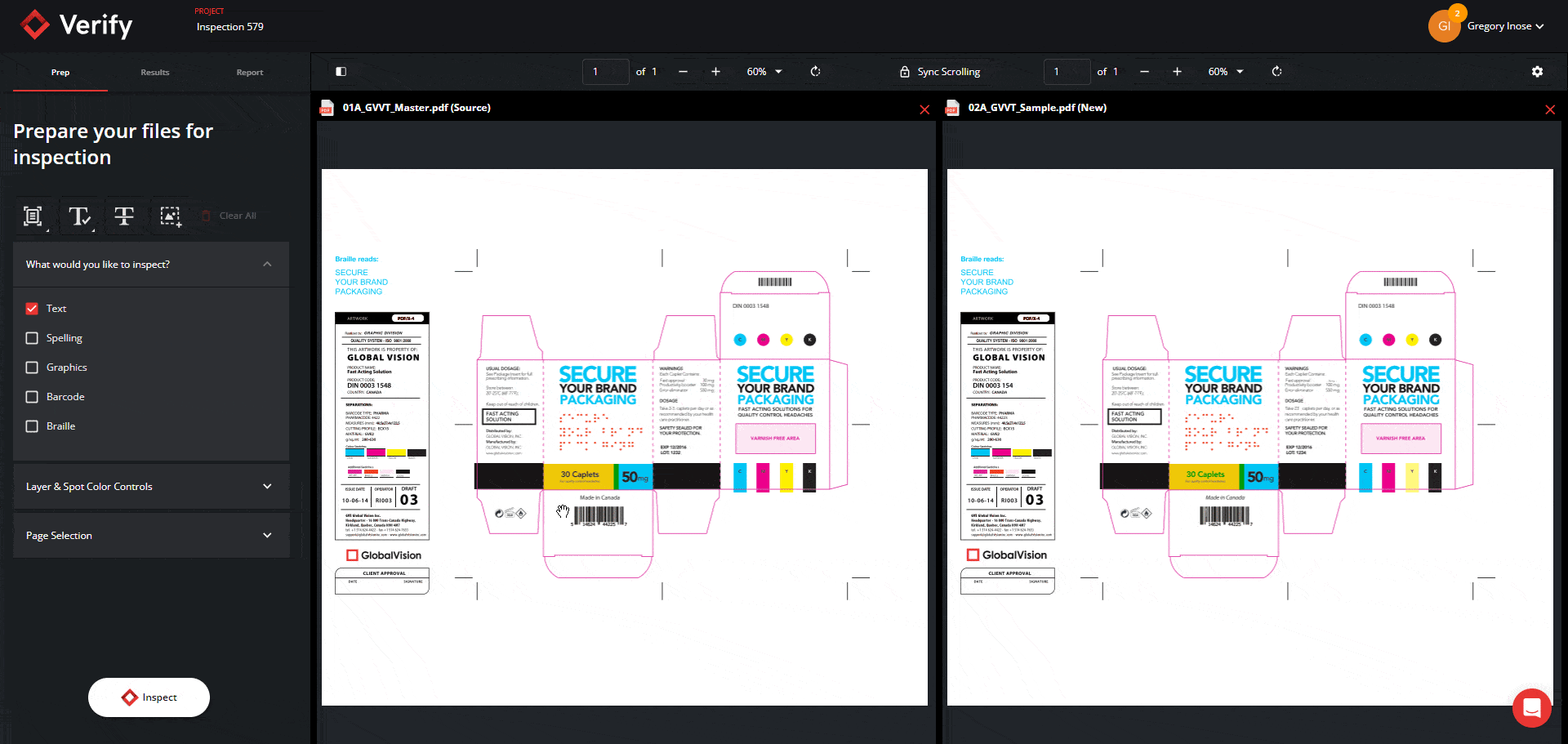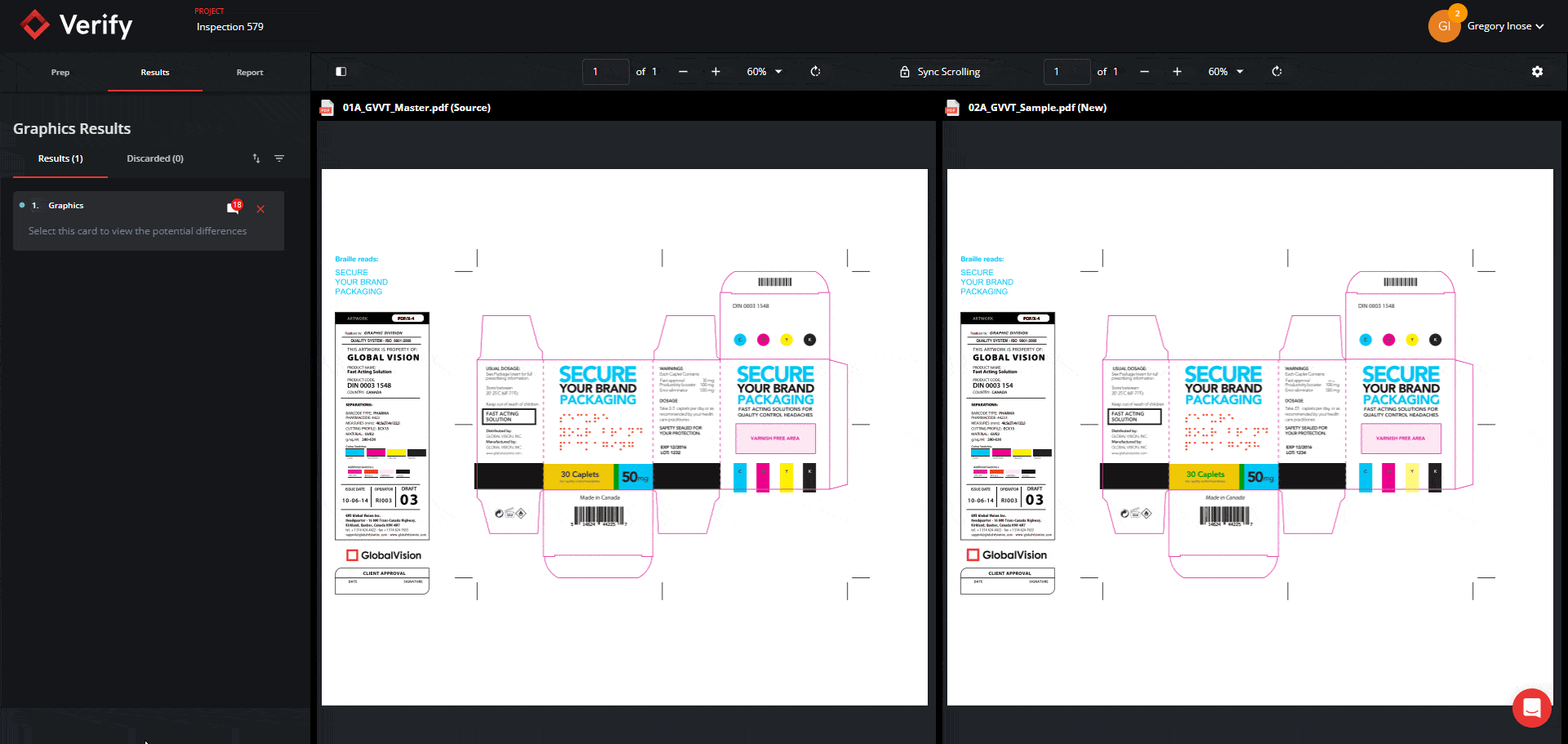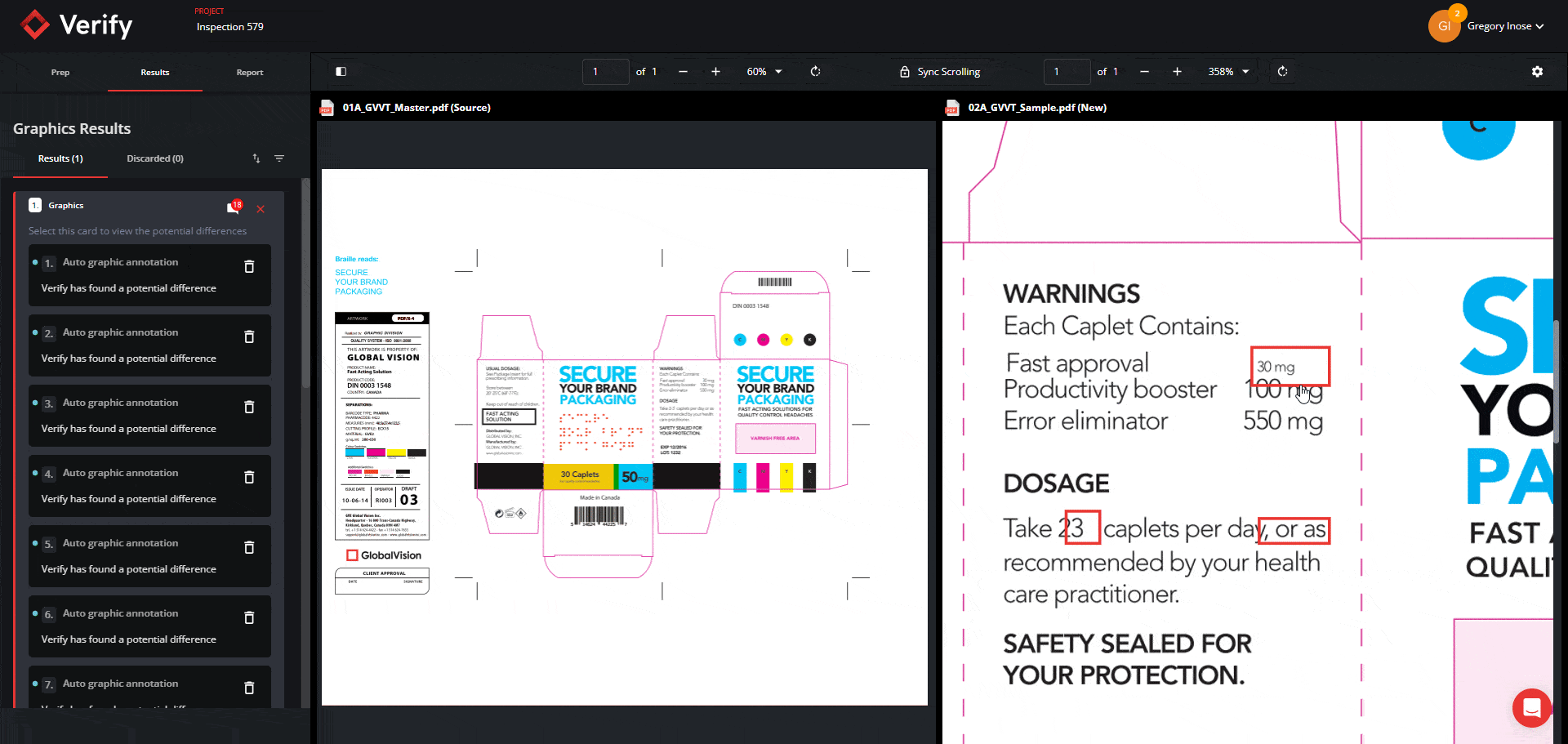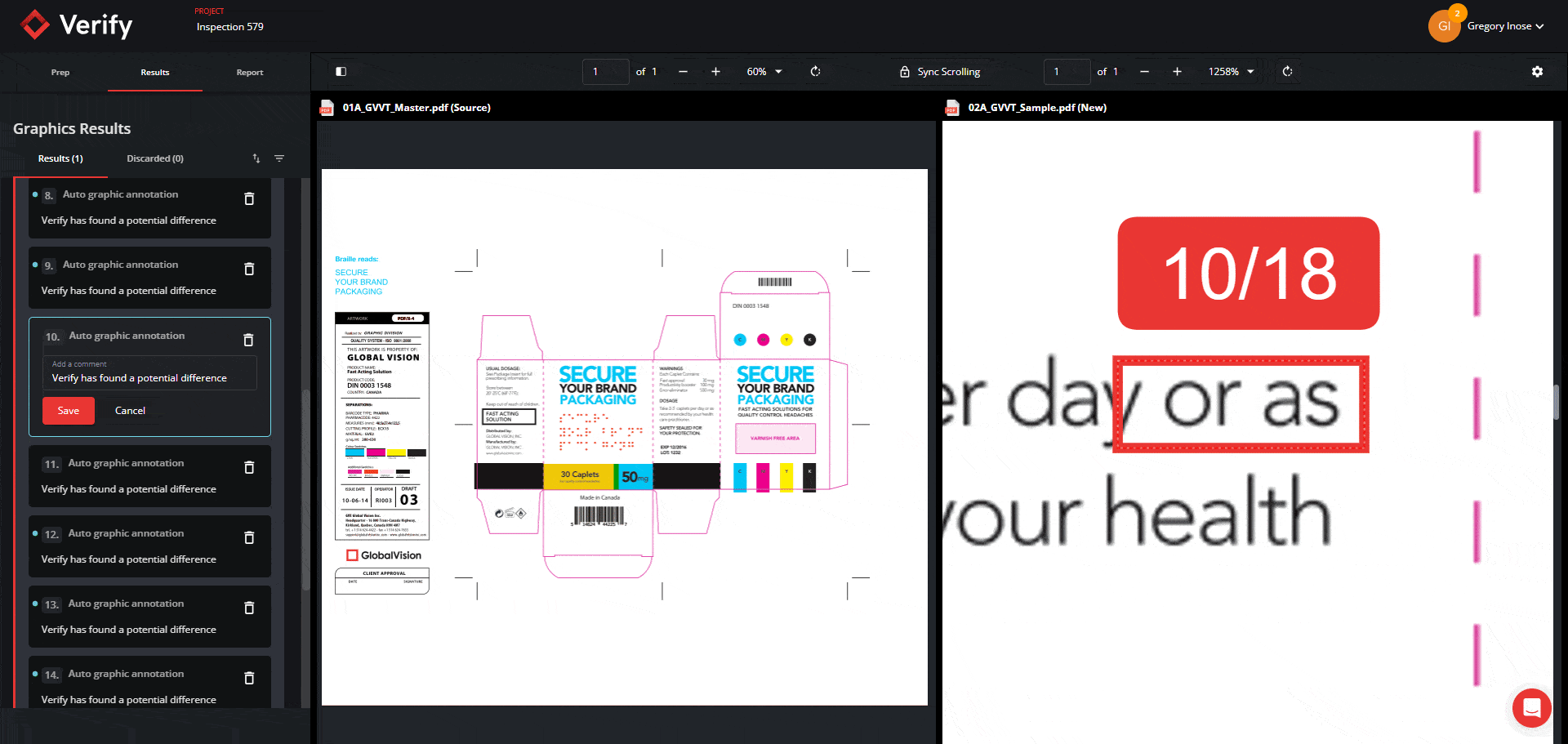
Graphics Inspection Captures Live Text Differences in Red Boxes
When running a graphics inspection, Verify will perform a pixel-for-pixel overlay, detecting all deviations, including deviations in live text, and will now capture them all within a red box.
By grouping and visualizing all differences found, this feature ultimately reduces confusion about which elements are being inspected when enabling Full Page Graphics.
See the new Graphics Inspection Improvements below
Scale Globally With Improved Accuracy of Asian and Right-to-Left Language Text Inspection
Multinational brands and agencies need to adhere to the compliance requirements of global authorities of the countries where their products and content are distributed, and are often faced with inspecting languages such as Asian text and right-to-left texts, such as Arabic and Hebrew, on various types of documents.
Without adequate processes and solutions, these companies are forced to either outsource these tasks or perform the inspections manually. This can lead to slow revision processes, delayed product-to-market times, and can ultimately hinder a company’s ability to scale its brand globally.
We improved our text inspection algorithm that recognizes and compares Asian characters and Right-to-Left languages. This allows multinationals to render more accurate inspection results and consequently, improve the quality of their multilingual documentation and packaging assets.
Reduce Unwanted Spelling Results
The Winter Release also brings new improvements to the spelling inspection algorithm and now offers a more reliable dictionary, rendering more accurate results for the following:
- Hyphenated words
- Soft-hyphenated words
- Words that include special characters
- Commonly used abbreviations
This is particularly beneficial for global brands and agencies that need to ensure all of their distributed content is accurate and free from all errors. Much like with multilingual documentation, without proper processes and software to help with these quality control tasks, international brands and companies may need to outsource tasks or do inspections manually. This can slow down revision times, delay product launches, and hinder global brand expansion.
Verify addresses these problems and offers a comprehensive and seamless solution to all organizations in a wide range of markets who want to ensure the quality of their products and brand.
See the new Reduced Unwanted Spelling Results feature below
Verify for Improved Proofreading
Verify’s Winter Release marks a significant advancement for cloud-based automated proofreading. Not only does it set new standards for precision, efficiency, and compliance, but also the refinement of OCR capabilities, streamlined custom dictionary workflows, revamped filtering tools, and enhanced graphics inspection features demonstrates GlobalVision’s commitment to delivering a seamless and accurate proofreading experience.
These innovations will elevate the quality of document inspections but also address specific challenges faced by diverse sectors, from regulated industries such as pharmaceuticals and print and packaging to creative sectors such as marketing agencies.
With a focus on reducing manual processes, improving inspection accuracy, and catering to the process complexities of global corporations, Verify empowers users to enhance the quality of their critical documentation. Our Winter Release not only meets but exceeds the evolving needs of regulated industries, making automated proofreading more accessible, efficient, and reliable.
To explore and leverage these cutting-edge features, book a demo of Verify and see firsthand the transformative impact it will have on your proofreading and quality control processes.
Customer Spotlight: IPG Health Leverages New Verify Features to Further Improve Proofreading Efficiency
IPG Health, a renowned network of agencies focused on health communication and marketing, serves top global pharmaceutical, biotech, and life sciences companies.
 For over a decade, IPG Health has leveraged GlobalVision products as part of their editorial processes to help ensure the integrity and quality of their clients’ content. With the introduction of notable workflow improvements, Verify’s Winter Release further enhances the efficiency of their proofreading.
For over a decade, IPG Health has leveraged GlobalVision products as part of their editorial processes to help ensure the integrity and quality of their clients’ content. With the introduction of notable workflow improvements, Verify’s Winter Release further enhances the efficiency of their proofreading.
More specifically, the new approval workflow for custom dictionaries — based in part on significant feedback from the IPG Health Editorial team — streamlines their spell check operations, and the newly added filtering options and improved graphics inspection accelerate their proofreading reviews while helping to ensure accuracy.
IPG Health is one among the many global organizations and life sciences companies leveraging Verify’s latest innovations!