Date: October, 2023 | Category: Compliance | Author: Hana Trokic
In the digital age, where data serves as the backbone of decision-making, business operations, and technological advancements, ensuring the integrity of data has become paramount.
From sensitive information to critical corporate records and beyond, the accuracy, consistency, and reliability of data play a pivotal role in building trust, driving innovation, and avoiding potentially catastrophic consequences.
This comprehensive guide is designed to unravel the intricate layers of data integrity, offering insights into its significance, the challenges it poses, and, most importantly, the proven strategies and best practices that individuals and organizations can adopt to safeguard their data.
Whether you’re seeking to refine your enterprise’s practices, you’re a small company that is eager to improve its systems and security, or personally looking into how you can secure your data and privacy, this guide is your compass in navigating the complex landscape of data integrity assurance.
What is Data Integrity?
At its core, data integrity represents the steadfast accuracy and consistency of information throughout its lifecycle. It encompasses the assurance that data can be either valid, invalid or in the process of becoming valid.
Error-checking and validation processes are methods often used to ensure data integrity and it’s easier to think of it as the trustworthiness of a digital asset, where any modifications, corruptions, or unauthorized changes are effectively prevented or quickly detected and corrected.
In essence, data integrity is the pillar on which sound decision-making, seamless processes, and reliable systems are built. It not only guards against accidental errors and technical glitches but also shields against intentional tampering, ensuring that data retains its value and authenticity, fostering confidence among stakeholders and enabling a strong foundation for operational excellence.
The key to a successful software development lifecycle? Data integrity. Read more here!
Why is Data Integrity Important?
Data integrity is a cornerstone of modern information-driven landscapes, playing a pivotal role in upholding the credibility and effectiveness of any data-driven endeavor.
In a world where organizations and individuals rely on data for critical decision-making, strategic planning, and innovation, the importance of data integrity cannot be overstated.
Maintaining data in its accurate, unaltered state ensures that insights drawn and actions taken are based on a foundation of truth, enhancing the reliability of outcomes. Moreover, data integrity is a foundation of regulatory compliance, particularly in industries handling sensitive information such as healthcare and finance, for example.
Without robust data integrity measures, the risk of errors slipping through, compromised security, and a damaged brand image pose a large threat. By creating a culture of data integrity, organizations foster trust among stakeholders and customers while driving efficiency in operations and securing their ability to navigate the complexities of the digital age with confidence.
Data Integrity Best Practices
When it comes to ensuring data integrity, there are several fundamental principles that serve as the foundation for maintaining the reliability and trustworthiness of your data.
These principles are collectively known as ALCOA, an acronym that stands for Attributable, Legible, Contemporaneous, Original, and Accurate.
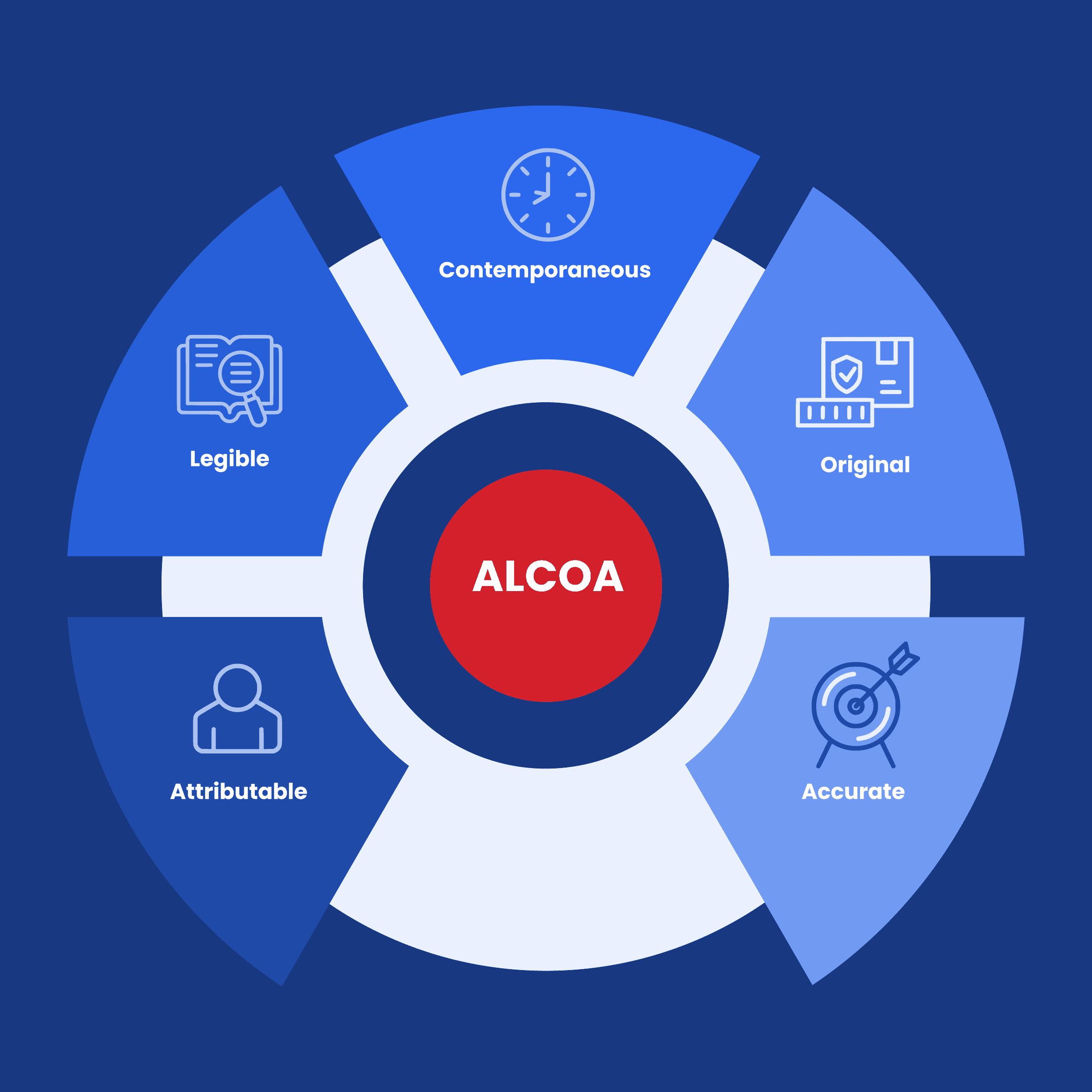
- Attributable: Data should clearly demonstrate who observed and recorded it when it was observed and recorded, and who it is about. This principle ensures transparency and accountability in data collection and management. When data is attributable, it becomes possible to trace its origins and understand the context in which it was generated.
- Legible: Data should be easy to understand and recorded permanently and original entries should be preserved. Legibility is crucial because it ensures that data can be effectively reviewed, analyzed, and shared without ambiguity.
- Contemporaneous: Data should be recorded as it was observed, and at the time it was executed. Timeliness in data recording is essential to prevent inaccuracies or omissions that can occur when data is retroactively documented.
- Original: Source data should be accessible and preserved in its original form. This ensures that data remains unaltered and unmanipulated throughout its lifecycle. Original data serves as a reference point for audits, validations, and quality assessments.
- Accurate: Data should be free from errors, and conform with the protocol. Accuracy is at the core of data integrity, as incorrect data can lead to faulty decisions, jeopardizing product quality, patient safety, and regulatory compliance.
What Are Threats to Data Integrity?
The landscape of data integrity faces a multitude of threats that can compromise the accuracy, reliability, and trustworthiness of information. Malicious cyberattacks, ranging from hacking and phishing to ransomware, pose a significant danger by potentially altering or stealing data.
System glitches, hardware failures, and software bugs can inadvertently introduce errors and inconsistencies. Data entry mistakes, often attributed to human error, can distort information at the point of creation.
Additionally, as data travels through various stages of its lifecycle, data integrity risks emerge during storage, transmission, and processing. Moreover, inadequate data governance and insufficient security measures can create vulnerabilities that malicious actors exploit. 
These threats collectively highlight the necessity of robust data integrity measures that encompass technological safeguards, employee training, vigilant monitoring, and stringent compliance with established protocols.
Overall, threats to data integrity can appear in many different forms. Some of the most common threats, however, are often internal. Examples include:
- Human error
- Unintended actions
- Security errors
- Malware
- Compromised hardware
How to Minimize Data Integrity Risks
In today’s marketplace, individuals and companies need to feel confident that there is no loss of quality when using computer systems. To accomplish this, there are effective strategies that companies may implement to manage their data integrity risks and ensure their data respects the ALCOA principle. By moving from a reactive to a proactive way of thinking, the following key requirements and controls may be put in place to ensure data integrity and minimize risks.
For more information about how you or your organization can ensure complete data integrity, read our detailed blog post.
Top Backup Strategies to Keep Data Integrity Intact
Just backing up data isn’t enough to keep data intact anymore. While it’s a necessary step and a good start, a backup means little if there aren’t adequate safeguards in place to protect the integrity of the data that might have to be recovered.
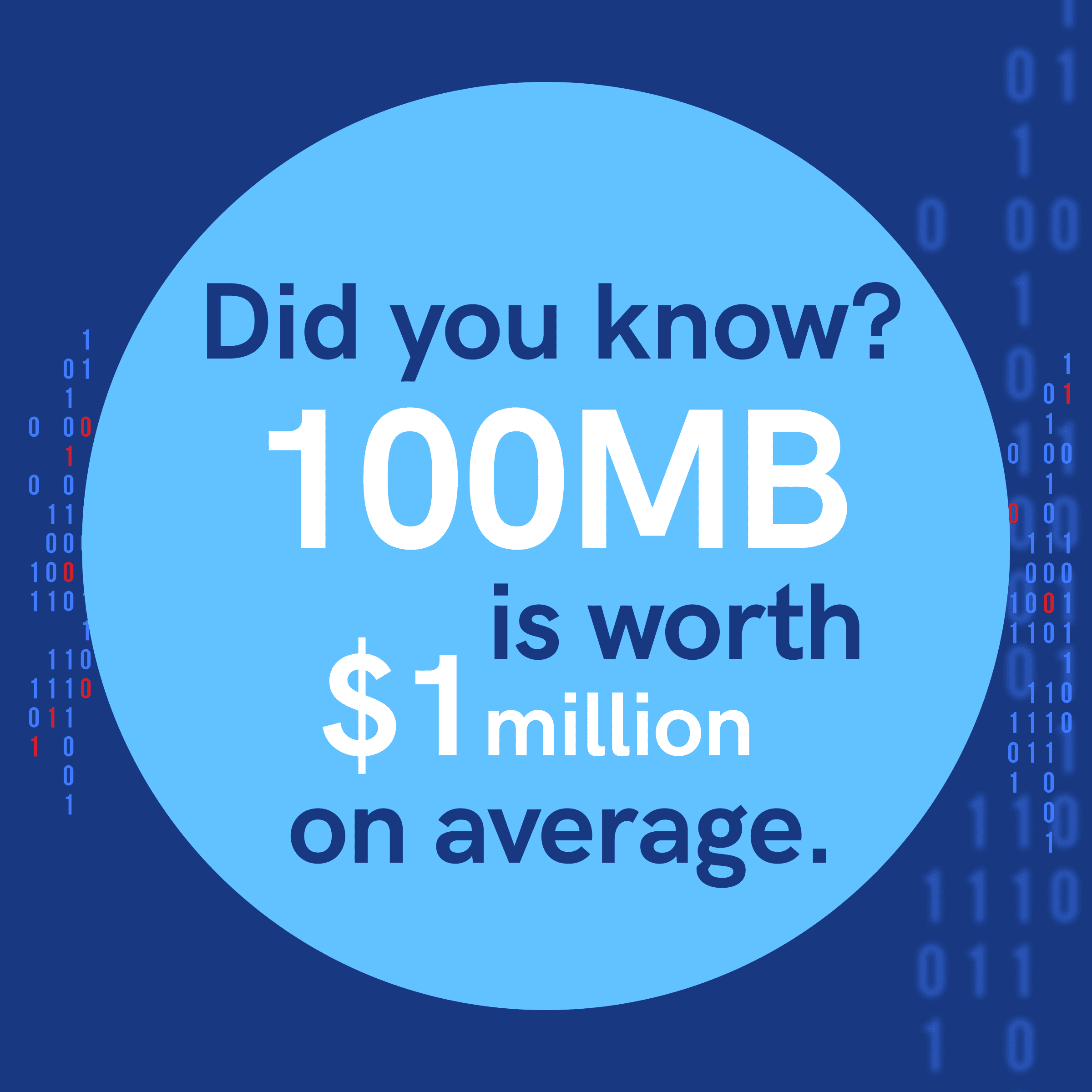
Even if backups themselves are a safeguard, they must be seen as an asset in their own right. After all, it’s estimated that 100 MB of company data is worth $1 million on average. Half the firms that cannot recover lost data in 10 days cannot recover themselves.
That is why, it is in every individual’s and company’s interest to have a good backup plan and strategy in place. Here are some backup strategies to keep data intact:
- Know What to Back Up: It’s not only files that must be backed up but programs, including Operating System software as well.
- Encrypt Your Back-Ups: Information must be kept secure for its data integrity to stay intact. One way to avoid confidential information from being compromised is to encrypt it into code.
- Make Regular Back-Ups: Ideally scheduled for when there is low network activity to prevent slowdown, back-ups should be made every day, with daily snapshots taken as well to monitor performance.
- Store Your Back-Ups Properly: It goes beyond storing your backups in environmentally controlled facilities. You would also want to store your backups off-site, separately from the original data.
To find out more about best practices and top-back-up strategies to keep your data integrity intact, read our in-depth blog post.
How to Reduce Data Integrity Risks for Regulated Industries
In regulated industries, reducing data integrity risks is paramount to ensure compliance and maintain public trust. By implementing stringent data management protocols, conducting regular audits, and fostering a culture of transparency and accountability, organizations can effectively mitigate data integrity risks and uphold regulatory standards.
Here are some ways businesses in regulated industries can overcome data integrity issues:
- Validate Input: Documented evidence of validation is a crucial way to ensure that input data is accurate. Once a data set is received, it should always be systematically verified.
- Validate Data: Ensuring data meets pre-determined specifications and key attributes is crucial to ensuring its validity and the system through which it passes.
- Remove Unnecessary Data: Duplicate files and unidentified data can potentially open doors to unwanted intruders set on exploiting or corrupting information.
- Data Back-Ups: Permanent data loss is a real threat to businesses. Staying on top of routine backup checks is crucial to avoid losing critical information. Businesses should be proactive in creating a recovery strategy in the event of an unexpected data loss or application error. This will help to restore any losses that could potentially occur.
- Manage Access Control: Setting limits and controls on who can access certain information within an organization is crucial in protecting data from unauthorized users, including intruders and impersonators. Actions must be taken to ensure that the unwanted spread of sensitive information is limited. Furthermore, the importance of physical access controls must not be neglected. Businesses should take care to protect places like server rooms that can be especially vulnerable to corruption.
- Traceability: Data integrity relies heavily on the ability to track down the source of a breach at any point within operations. Ensuring that audit trails are consistently in place helps to provide security in the event of a breach and allows organizations to identify the source.
For a step-by-step look at how you or your organization can ensure data integrity, read our in-depth “12 Ways to Reduce Data Integrity Risks for Regulated Industries”
The Role of Document Comparison Software in Ensuring Data Integrity
As automation technology becomes increasingly prevalent and the use of different software, applications, and the internet becomes an absolute necessity, data integrity is as important as ever. Not only does it serve as the driving force behind technology of all forms, but it also acts as a fail-safe.
Document comparison software is one viable way to catch and stop errors that risk corrupting data in their tracks. The software is designed to compare two or more documents to identify all differences, changes, and errors between them allowing for ease of proofreading, and editing and ultimately leading to error-free content. As previously mentioned, this type of software is particularly useful in industries that deal with highly regulated and critical content.
Here are some ways document comparison software can help ensure data integrity:
- Enhances security
- Reduces Human Error
- Prevents Issues from Recurring
Enhances Security
While data security is different than data integrity, the two go hand in hand. Like data quality, data security is a single facet of data integrity (but not vice versa). Nevertheless, without the proper degree of security, data can become compromised due to breaches, among other threats. In other words, for data to have integrity, it must first be secure.
As a result, document comparison software can be considered a key component of any complement of tools designed and implemented to enhance the security of data. Errors are simply outliers or anomalies, which are defined as observations that lie outside of norms Document comparison software can build baselines of systems, their users, and the data they create, leading to the easy detection of behavioral deviations, whether there is malicious intent or not.
Do you think following FDA data integrity guidelines is hard? It’s way easier than you think!
Reduces Human Error
There’s an inherent risk whenever you rely on human resources. There are some things a machine will likely never be able to do as well, but analyzing data is not one of them. It’s similar to the situation with manual proofreading, where, the longer the process is, the less likely errors are to get caught. Fatigue sets in eventually and the effectiveness of proofreaders declines over time.
In much the same way, the automated analysis of unstructured data saves time, thereby improving the overall efficiency of the process. Employees wouldn’t be replaced, either. There would still be a need to oversee the analysis. The right document comparison software would all the while keep relevant parties apprised of how the data behaves.
Interested in learning more about how document comparison software can help ensure data integrity? Read more here!
Prevents Issues from Recurring
It isn’t just the current errors the software might catch, but the ones in the future that would otherwise slip through the cracks. Consider document comparison software as an example. A form of error-detection software, GlobalVision features an audit trail for compliance with FDA 21 CFR Part 11.
The platform doesn’t just go over the document pixel-by-pixel or character-by-character to detect graphics and text differences (among other types). The application tracks parameter changes and log-ins, so data becomes “attributable,” one of the five principles of data integrity.
Read about how GlobalVision Adds Electronic Signatures for Enhanced Data Integrity to the Most Comprehensive Platform Yet!
The Importance of an Audit Trail to Securing Data Integrity
Though the word might spring negative connotations, audits are an unavoidable part of life for many corporations that bring many benefits to daily functions and processes.
In essence, trails are the lists of transactions or events kept track of to help auditors and, in many ways, those being audited as well. An audit is simply an investigation of accounts and records in general.
For example, audits can be key to achieving and maintaining regulatory compliance, which is in turn critical to operating in regulatory sectors. It is also important to note that external and internal audits are to be expected on a regular basis.
External vs. Internal Audits
External audits and internal audits serve different purposes:
- External audits: done by independent third parties and focused on confirming accurate financial statements for regulators and stakeholders.
- Internal audits: performed by the organization’s own team, and aims to assess and improve internal controls, risk management, operations, and adherence to policies.
The Benefits of an Audit Trail
Regardless of the focus of an audit, trials are undeniably critical to their success. And success is what all parties should strive for, whether they’re doing the auditing or being audited.

Audits can be made easier if all required records have been kept and are easily accessible to auditors. Automated trails that are easily searchable make smooth audits more of a reality.
Trails are theoretically included in the software as one of many required technical controls that enable users to achieve compliance with 21 CFR Part 11 with the Food and Drug Administration (in the United States; equivalent to Annex 11 in the European Union).
Compliance here ensures companies implement good business practices through reliable electronic records, which must be able to be accurately displayed and exported. Here, the audit trail serves to log what changes to application data were made, when, and by whom and be available for review.
Whoever ends up conducting that review, whether it’s an agency or the company itself, the auditor will no doubt thank you as the bigger picture begins to take shape. Identifying the individual trees is key to seeing the forest as a whole, though. Finding your way through can be hard, but an audit trail can clearly reveal the right path to take.
Read more about the importance of an audit trail to securing data integrity here.
Step Up Your Data Security
Data integrity serves as the pillar for informed decision-making, operational efficiency, and regulatory compliance.
By delving into the significance of data integrity, the principles of ALCOA, threats to data security, and practical strategies for safeguarding it, this guide equips individuals and organizations with the knowledge and tools necessary to protect their data assets.
Whether you are an individual seeking to secure your personal information or a business aiming to strengthen your operations and brand, the principles and practices outlined provide a compass to navigate the complexities of the digital age with confidence.
As data continues to evolve and become more integral to our lives, the assurance of its integrity remains vital, ensuring that our digital assets are built on strong foundations of truth, trust, and reliability.
Ready to upgrade your data security? Take a step in the right direction by setting up proper processes through automated quality control. Request a demo of our innovative proofreading software and see how this technology can revolutionize your everyday business practices along with your data security.
_________________________________________________________________________________________
Did you enjoy reading this post? Check out these other related post:

Enhancing Your Verify Experience – Our Fall Release is Here
Date: October, 2023 | Category: Company | Author: Hana Trokic
Are you ready to heighten your Verify inspections and overall proofreading experience?
We’re thrilled to introduce significant improvements to Verify, designed to make the automated proofreading experience easier and more efficient. These product advancements have been rolled out in two waves throughout September and October!
With a substantial performance upgrade, a 69% improvement in Spell Check results and the addition of Single File Inspections, now called ‘Proofreader Mode’, our Verify Fall Release ensures your content is perfected, faster.
Additionally, this release marks a huge milestone for Verify’s core technology, as it has been completely rehauled and updated to an entirely new architecture with all modern technology—get ready to elevate your compliance review and inspection processes!
The Verify Fall Release also brings a number of major improvements and features including:
If you want to learn more about how to leverage these new features, book a demo of Verify here.
Upgraded Architecture, Upgraded Inspections
We’ve upgraded to an entirely new architecture with modern technology to offer you the best possible proofreading experience on the market. Though this upgrade brings with it many benefits and advantages, possibly the largest benefit is that it will increase development speed, resulting in highly requested features being released more rapidly.
This accelerated development pace translates to a high turnover of new features and capabilities, allowing regulatory and commercial experts to stay ahead of evolving compliance requirements by having the best tools available to them during their quality assurance processes.
The upgraded architecture also increases Verify’s reliability and uptime. This results in fewer interruptions to critical processes, where you can rest assured that your workflows run as smoothly as possible and with the utmost ease. In the exceptional instance of an issue occurring, the enhanced infrastructure ensures swift resolution, minimizing downtime and enhancing productivity.
Moreover, the optimization of our algorithms for faster inspections enables regulatory professionals and beyond to expedite their reviews, ensuring that all documents and critical content adhere to compliance standards with greater efficiency and precision.
This major breakthrough in Verify’s development empowers regulatory professionals and all automated proofreading users to work more effectively, maintain compliance, and improve the overall quality and reliability of their operations.
Boosted Responsiveness For Heightened day-to-day Efficiency.
Time is crucial when inspecting documents, especially for regulated industries where manual compliance procedures can sometimes hinder time-to-market
This new release brings a remarkable 15% improvement in responsiveness, backed by a more stable infrastructure to further help accelerate the revision cycle of critical content and ensure error-free files in record time.
Through this new upgrade, you can expect to load and inspect large files faster than ever, without rendering issues, decreasing delays in inspections and allowing for an overall more seamless inspection.
Save More Time With Built-in Spell Check Rules
The new fall release also brings with it new built-in rules that improve Spell-Check results by 69%. The new update will only flag important spelling errors, ensuring a more efficient review process.
This means the software no longer detects terms such as URLs or words containing numbers as spelling errors allowing teams to focus their energy on more crucial aspects of their inspections without getting set back by minor details.
The improved Spell Check feature also assists regulatory professionals in meeting FDA requirements by ensuring accurate, clear, and error-free documents. This helps maintain compliance with FDA guidelines, reduces legal and reputational risks, and promotes efficiency.
Single File Inspection For Ease and Simplicity
We’re simplifying the inspection process with the introduction of a Single File Inspection capability called “Proofreader Mode”. This feature allows you to upload and inspect a single file when there is no need to compare it against a source file. This feature is particularly beneficial for regulatory professionals who have many documents to review and need to optimize their entire process.
The upgrade was designed to facilitate Barcode Inspections and Spell Checks, and Braille Inspection. Previously, the software required that two identical files be uploaded to perform an inspection. Now, you can tackle these tasks with precision and ease, one file at a time.
More Review Enhancing Features
If those upgrades are not enough to bring your compliance reviews to the next level, regulatory and commercial professionals can expect to enhance their inspections through the following features:
A Better Verify Experience
With Verify’s fall release and a complete overhaul of our core technology, you will experience a more responsive and stable platform. With the addition of the many features and functionality presented, regulatory and commercial professionals tasked with reviewing content for total compliance lifesciences can perfect their content, faster.
These exciting updates to Verify are a testament to our commitment to improving the overall user experience and making quality inspections more efficient and effective.
For more detailed information about our latest upgrades, read the Release Notes, and, make sure to watch our informative video, which highlights some of the new additions to the Verify fall release.
If you haven’t experienced the time-saving and compliance enhancing powers of Verify yet, get started today with a Free Trial.
A Definitive Guide to Data Integrity Assurance
Date: October, 2023 | Category: Compliance | Author: Hana Trokic
In the digital age, where data serves as the backbone of decision-making, business operations, and technological advancements, ensuring the integrity of data has become paramount.
From sensitive information to critical corporate records and beyond, the accuracy, consistency, and reliability of data play a pivotal role in building trust, driving innovation, and avoiding potentially catastrophic consequences.
This comprehensive guide is designed to unravel the intricate layers of data integrity, offering insights into its significance, the challenges it poses, and, most importantly, the proven strategies and best practices that individuals and organizations can adopt to safeguard their data.
Whether you’re seeking to refine your enterprise’s practices, you’re a small company that is eager to improve its systems and security, or personally looking into how you can secure your data and privacy, this guide is your compass in navigating the complex landscape of data integrity assurance.
What is Data Integrity?
At its core, data integrity represents the steadfast accuracy and consistency of information throughout its lifecycle. It encompasses the assurance that data can be either valid, invalid or in the process of becoming valid.
Error-checking and validation processes are methods often used to ensure data integrity and it’s easier to think of it as the trustworthiness of a digital asset, where any modifications, corruptions, or unauthorized changes are effectively prevented or quickly detected and corrected.
In essence, data integrity is the pillar on which sound decision-making, seamless processes, and reliable systems are built. It not only guards against accidental errors and technical glitches but also shields against intentional tampering, ensuring that data retains its value and authenticity, fostering confidence among stakeholders and enabling a strong foundation for operational excellence.
The key to a successful software development lifecycle? Data integrity. Read more here!
Why is Data Integrity Important?
Data integrity is a cornerstone of modern information-driven landscapes, playing a pivotal role in upholding the credibility and effectiveness of any data-driven endeavor.
In a world where organizations and individuals rely on data for critical decision-making, strategic planning, and innovation, the importance of data integrity cannot be overstated.
Maintaining data in its accurate, unaltered state ensures that insights drawn and actions taken are based on a foundation of truth, enhancing the reliability of outcomes. Moreover, data integrity is a foundation of regulatory compliance, particularly in industries handling sensitive information such as healthcare and finance, for example.
Without robust data integrity measures, the risk of errors slipping through, compromised security, and a damaged brand image pose a large threat. By creating a culture of data integrity, organizations foster trust among stakeholders and customers while driving efficiency in operations and securing their ability to navigate the complexities of the digital age with confidence.
Data Integrity Best Practices
When it comes to ensuring data integrity, there are several fundamental principles that serve as the foundation for maintaining the reliability and trustworthiness of your data.
These principles are collectively known as ALCOA, an acronym that stands for Attributable, Legible, Contemporaneous, Original, and Accurate.
What Are Threats to Data Integrity?
The landscape of data integrity faces a multitude of threats that can compromise the accuracy, reliability, and trustworthiness of information. Malicious cyberattacks, ranging from hacking and phishing to ransomware, pose a significant danger by potentially altering or stealing data.
System glitches, hardware failures, and software bugs can inadvertently introduce errors and inconsistencies. Data entry mistakes, often attributed to human error, can distort information at the point of creation.
Additionally, as data travels through various stages of its lifecycle, data integrity risks emerge during storage, transmission, and processing. Moreover, inadequate data governance and insufficient security measures can create vulnerabilities that malicious actors exploit.
These threats collectively highlight the necessity of robust data integrity measures that encompass technological safeguards, employee training, vigilant monitoring, and stringent compliance with established protocols.
Overall, threats to data integrity can appear in many different forms. Some of the most common threats, however, are often internal. Examples include:
How to Minimize Data Integrity Risks
In today’s marketplace, individuals and companies need to feel confident that there is no loss of quality when using computer systems. To accomplish this, there are effective strategies that companies may implement to manage their data integrity risks and ensure their data respects the ALCOA principle. By moving from a reactive to a proactive way of thinking, the following key requirements and controls may be put in place to ensure data integrity and minimize risks.
For more information about how you or your organization can ensure complete data integrity, read our detailed blog post.
Top Backup Strategies to Keep Data Integrity Intact
Just backing up data isn’t enough to keep data intact anymore. While it’s a necessary step and a good start, a backup means little if there aren’t adequate safeguards in place to protect the integrity of the data that might have to be recovered.
Even if backups themselves are a safeguard, they must be seen as an asset in their own right. After all, it’s estimated that 100 MB of company data is worth $1 million on average. Half the firms that cannot recover lost data in 10 days cannot recover themselves.
That is why, it is in every individual’s and company’s interest to have a good backup plan and strategy in place. Here are some backup strategies to keep data intact:
To find out more about best practices and top-back-up strategies to keep your data integrity intact, read our in-depth blog post.
How to Reduce Data Integrity Risks for Regulated Industries
In regulated industries, reducing data integrity risks is paramount to ensure compliance and maintain public trust. By implementing stringent data management protocols, conducting regular audits, and fostering a culture of transparency and accountability, organizations can effectively mitigate data integrity risks and uphold regulatory standards.
Here are some ways businesses in regulated industries can overcome data integrity issues:
For a step-by-step look at how you or your organization can ensure data integrity, read our in-depth “12 Ways to Reduce Data Integrity Risks for Regulated Industries”
The Role of Document Comparison Software in Ensuring Data Integrity
As automation technology becomes increasingly prevalent and the use of different software, applications, and the internet becomes an absolute necessity, data integrity is as important as ever. Not only does it serve as the driving force behind technology of all forms, but it also acts as a fail-safe.
Document comparison software is one viable way to catch and stop errors that risk corrupting data in their tracks. The software is designed to compare two or more documents to identify all differences, changes, and errors between them allowing for ease of proofreading, and editing and ultimately leading to error-free content. As previously mentioned, this type of software is particularly useful in industries that deal with highly regulated and critical content.
Here are some ways document comparison software can help ensure data integrity:
Enhances Security
While data security is different than data integrity, the two go hand in hand. Like data quality, data security is a single facet of data integrity (but not vice versa). Nevertheless, without the proper degree of security, data can become compromised due to breaches, among other threats. In other words, for data to have integrity, it must first be secure.
As a result, document comparison software can be considered a key component of any complement of tools designed and implemented to enhance the security of data. Errors are simply outliers or anomalies, which are defined as observations that lie outside of norms Document comparison software can build baselines of systems, their users, and the data they create, leading to the easy detection of behavioral deviations, whether there is malicious intent or not.
Do you think following FDA data integrity guidelines is hard? It’s way easier than you think!
Reduces Human Error
There’s an inherent risk whenever you rely on human resources. There are some things a machine will likely never be able to do as well, but analyzing data is not one of them. It’s similar to the situation with manual proofreading, where, the longer the process is, the less likely errors are to get caught. Fatigue sets in eventually and the effectiveness of proofreaders declines over time.
In much the same way, the automated analysis of unstructured data saves time, thereby improving the overall efficiency of the process. Employees wouldn’t be replaced, either. There would still be a need to oversee the analysis. The right document comparison software would all the while keep relevant parties apprised of how the data behaves.
Interested in learning more about how document comparison software can help ensure data integrity? Read more here!
Prevents Issues from Recurring
It isn’t just the current errors the software might catch, but the ones in the future that would otherwise slip through the cracks. Consider document comparison software as an example. A form of error-detection software, GlobalVision features an audit trail for compliance with FDA 21 CFR Part 11.
The platform doesn’t just go over the document pixel-by-pixel or character-by-character to detect graphics and text differences (among other types). The application tracks parameter changes and log-ins, so data becomes “attributable,” one of the five principles of data integrity.
Read about how GlobalVision Adds Electronic Signatures for Enhanced Data Integrity to the Most Comprehensive Platform Yet!
The Importance of an Audit Trail to Securing Data Integrity
Though the word might spring negative connotations, audits are an unavoidable part of life for many corporations that bring many benefits to daily functions and processes.
In essence, trails are the lists of transactions or events kept track of to help auditors and, in many ways, those being audited as well. An audit is simply an investigation of accounts and records in general.
For example, audits can be key to achieving and maintaining regulatory compliance, which is in turn critical to operating in regulatory sectors. It is also important to note that external and internal audits are to be expected on a regular basis.
External vs. Internal Audits
External audits and internal audits serve different purposes:
The Benefits of an Audit Trail
Regardless of the focus of an audit, trials are undeniably critical to their success. And success is what all parties should strive for, whether they’re doing the auditing or being audited.
Audits can be made easier if all required records have been kept and are easily accessible to auditors. Automated trails that are easily searchable make smooth audits more of a reality.
Trails are theoretically included in the software as one of many required technical controls that enable users to achieve compliance with 21 CFR Part 11 with the Food and Drug Administration (in the United States; equivalent to Annex 11 in the European Union).
Compliance here ensures companies implement good business practices through reliable electronic records, which must be able to be accurately displayed and exported. Here, the audit trail serves to log what changes to application data were made, when, and by whom and be available for review.
Whoever ends up conducting that review, whether it’s an agency or the company itself, the auditor will no doubt thank you as the bigger picture begins to take shape. Identifying the individual trees is key to seeing the forest as a whole, though. Finding your way through can be hard, but an audit trail can clearly reveal the right path to take.
Read more about the importance of an audit trail to securing data integrity here.
Step Up Your Data Security
Data integrity serves as the pillar for informed decision-making, operational efficiency, and regulatory compliance.
By delving into the significance of data integrity, the principles of ALCOA, threats to data security, and practical strategies for safeguarding it, this guide equips individuals and organizations with the knowledge and tools necessary to protect their data assets.
Whether you are an individual seeking to secure your personal information or a business aiming to strengthen your operations and brand, the principles and practices outlined provide a compass to navigate the complexities of the digital age with confidence.
As data continues to evolve and become more integral to our lives, the assurance of its integrity remains vital, ensuring that our digital assets are built on strong foundations of truth, trust, and reliability.
Ready to upgrade your data security? Take a step in the right direction by setting up proper processes through automated quality control. Request a demo of our innovative proofreading software and see how this technology can revolutionize your everyday business practices along with your data security.
_________________________________________________________________________________________
Did you enjoy reading this post? Check out these other related post:
OCR: Everything You Need to Know About Optical Character Recognition
Date: September, 2023 | Category: Proofreading | Author: Hana Trokic
Optical Character Recognition (OCR), is a technology that has revolutionized how we interact with text. This technology enables computers to decipher and manipulate printed, handwritten, and images of text, from an array of sources, including digital files, scanned documents, web pages, and more.
In this blog post, we’ll look into the fundamentals of Optical Character Recognition, explore the distinctions between live, rasterized, and vectorized text, and discover its versatile applications across various industries.
Read on to find out everything you need to know about OCR’s potential and how it can benefit your specific use case.
What is Optical Character Recognition?
OCR, short for Optical Character Recognition, is a transformative technology that converts printed, handwritten text or images into machine-encoded text, otherwise known as live text. It allows computers to recognize, understand, and manipulate text from various sources.
The primary goal of Optical Character Recognition is to make text more accessible and editable, enabling users to extract valuable information from physical documents or images and convert it into a digital, searchable format. Besides live text, text can also be rasterized or vectorized which makes the need for OCR crucial when editing digital assets and documents.
It is also important to note that OCR is otherwise a field of AI that focuses on recognizing and extracting text from images without live text. While Optical Character Recognition itself is a specific application within AI, it relies on various AI techniques and algorithms to perform its tasks such as machine learning.
Difference Between Live, Rasterized, and Vectorized Text
Knowing the difference between live, rasterized, and vectorized text is important in various contexts, especially when working with digital designs, graphics, and prints.
Here is a simple breakdown to help you understand their meanings and main differences:
Optical Character Recognition For Different Use Cases
Now that we understand the difference in text types, it’s important to understand how Optical Character Recognition can benefit users in real-life scenarios. Optical Character Recognition technology is valuable in a wide range of industries and applications where converting printed, handwritten text, and images into machine-readable digital text is essential.
This is especially useful in regulated industries and print and packaging during the quality review and proofreading stages of the product life cycle. Highly regulated industries have little room for error in their critical content. As such, any inaccuracies in content can lead to catastrophic consequences such as product recalls or customer safety issues. The addition of OCR in the editing and revision stages enables errors to be caught and fixed before products go out to market.
Here is a detailed look at how Optical Character Recognition is beneficial in different use cases:
The Importance of Optical Character Recognition in Proofreading
When proofreading documents, it is best to ensure that all text is live text to ease the revision and editing process. If text is not live, and is instead rasterized or vectorized, it is best that your proofreading platform offers Optical Character Recognition capabilities to transform any and all text into live text.
Here are some reasons why OCR is important when proofreading your documents:
Handling Non-Live Text: One of the primary reasons Optical Character Recognition is crucial in proofreading documents is its ability to handle non-live text effectively. As non-live text is text that has been rendered as static images or part of an image, without OCR, proofreaders would face significant challenges in identifying and correcting errors in content. OCR’s capability to convert non-live text into dynamic, editable formats allows proofreaders to efficiently review and edit content that would otherwise be inaccessible or difficult to modify.
Streamlining Compliance Efforts: In industries where regulatory compliance is essential, OCR plays a vital role in streamlining proofreading processes. Many compliance-related documents contain non-live text, such as labels, warnings, packing, etc. making Optical Character Recognition crucial for ensuring the accuracy of critical content. By using OCR to extract, review, and edit content, organizations can reduce the risk of compliance errors, maintain adherence to legal standards, and minimize the associated costs and potential liabilities. Ultimately, this significantly reduces the risk of recalls and any non-compliance issues with FDA or other health authority requirements.
Enhancing Efficiency in Quality Control: Whether it’s labeling quality control or press quality control, Optical Character Recognition significantly enhances efficiency in many industries. In labeling quality control, where label proofs often consist of non-live text and graphics, OCR’s conversion of non-live text into editable formats simplifies the proofreading process. Similarly, in press quality control for printed materials, Optical Character Recognition technology helps identify typographical errors, formatting issues, or missing text. This efficiency not only saves time but also reduces the likelihood of costly printing errors and reprints, thereby enhancing the overall quality assurance process.
GlobalVision’s Verify and OCR
GlobalVision’s newest, most innovative cloud-based proofreading software, Verify, is currently developing and testing the platform’s Optical Character Recognition capabilities that allow users to inspect flattened text on documents such as promotional material screenshots and supplier proofs by converting the digital images into readable, live text format.
Verify’s OCR technology relies on machine learning which is a subset of artificial intelligence (AI) technology.
Verify, uses machine learning and computer vision algorithms to recognize characters and words in images or documents. It involves the use of computational methods to perform tasks that typically require human intelligence or manual work, such as reading and understanding text within images.
Because of its use of artificial intelligence, it’s important to note that Optical Character Recognition can never be perfect and there is always a chance of error. An example would be when detecting characters that are very similar such as “O” and “0”.
For a detailed overview of Verify’s OCR capabilities, watch our informational video.
Optical Character Recognition For Error-Free Content
Optical Character Recognition is a powerful technology that transforms non-live text from various sources, making it editable and accessible. It’s essential for proofreading as it can handle non-editable text, streamline compliance tasks, and improve quality control processes.
It is important to note that in most cases, it is best to follow best practices and create files with live text. For more information about how to follow these best practices, read Section 3 of our Artwork Creation Guide. However, sometimes we cannot avoid working files with non-live text making the need for Optical Character Recognition inevitable.
In these cases, it is best to turn to software to transform your non-live text documents, enable editing, and ease the complete revision process. GlobalVision’s Verify, along with its lightning-fast inspection capabilities and robust set of proofreading features, is developing its OCR capabilities to further strengthen inspection processes for those dealing with non-live text.
If you’re ready to delve into Verify’s many market-leading proofreading capabilities, get started today and try Verify for free!
GlobalVision is Going to RAPS Convergence 2023 in Montreal
Date: September, 2023 | Category: Company | Author: Hana Trokic
GlobalVision is thrilled to announce our participation in RAPS Convergence 2023, set to take place in Montreal from October 3rd to 5th. This event promises to be a pivotal networking and learning experience for all professionals in the regulatory industry and similar fields.
Join us at one of the top life sciences events of the year as we engage with industry leaders and professionals, highlighting our innovative proofreading solutions while fostering valuable connections. Make sure to stop by our booth #225 during the event to gain valuable insights into our products and services and see what’s new with GlobalVision!
Why RAPS Convergence 2023
RAPS Convergence is renowned for bringing together experts, innovators, and thought leaders in regulatory affairs and life sciences. It serves as a hub for networking, knowledge sharing, and exploring the latest advancements in regulatory science.
As GlobalVision is a market-leading proofreading software and a top choice for regulatory experts in quality assurance, we understand the significance of staying at the forefront of industry trends. That is why we are excited to showcase our products to an even broader audience, helping regulatory professionals drive efficiency across their entire proofreading workflows and ensuring flawless critical content.
What to Expect from GlobalVision at RAPS
As a market-leading quality control software and solution for regulated industries, GlobalVision is committed to helping organizations enhance their quality control processes. Our innovative technology helps ensure packaging, labeling, and all critical content are accurate and compliant with regulatory requirements.
At RAPS Convergence 2023, we will showcase our cutting-edge software, offer free demos to attendees, and showcase how our innovative products, including our newest and most innovative cloud-based software Verify, can streamline quality control processes, reduce errors, and save time and resources.
Engage with Our GlobalVision Experts
Our team of experts will be on hand at our booth to provide live demonstrations of our software and answer all questions. We value every opportunity to engage with professionals in the regulatory affairs community, and we’re eager to hear about the latest and most specific challenges and needs of the industry.
In addition to our booth presence, GlobalVision will also have an educational session and presentation during the conference. Our presentation “How AI Will Power the Future of Proofreading in Pharma” will cover the growing role of technology, specifically artificial intelligence, in the pharmaceutical and regulatory industries.
Finally, RAPS Convergence 2023 is an excellent platform to connect with like-minded professionals. That is why we are excited to use this opportunity to network with top industry experts, share experiences, and build connections that can help drive success in the regulatory affairs and life sciences industry.
Join Us in Montreal
Mark your calendars for October 3-5, 2023, and join us at RAPS Convergence in Montreal for an incredible opportunity to learn, network, and explore the latest innovations in regulatory affairs.
We can’t wait to meet you, showcase our solutions, and discuss how GlobalVision can empower your organization to achieve compliance and quality excellence. See you at RAPS Convergence 2023!
An Introduction to Automated Quality Control
Date: August, 2023 | Category: Quality | Author: Hana Trokic
Terms like “audit,” “inspection,” and “quality control” are prevalent in any manufacturing business. The first one refers to analyzing manufacturing organizations and processes, whereas the second and third refer to any product-checking activity.
Generally, quality inspectors do this by following a pre-established list based on certain product specifications. In fact, any type of product can be inspected, starting with just the components used for the product to semi-finished ones and (most often) the finished product itself.
According to the ISO 2859 standard – which is derived from MIL-STD 105 E – quality inspection involves activities such as testing, gauging, examining, or measuring one or more product characteristics.
These quality inspections are usually extremely time-consuming and tedious processes that leave little room for error, due to their critical nature of ensuring product quality and safety.
Thankfully, automated quality control is a cutting-edge technological approach that revolutionizes the way industries ensure product excellence, utilizing advanced systems and algorithms to enhance efficiency, accuracy, and consistency throughout the manufacturing revision processes.
Want to learn more about automation, proofreading, and quality control? Read more content from GlobalVision here!
Quality Inspections – Getting Started Early
Quality inspections in any product lifecycle should begin early.
Starting quality inspections early in the product development lifecycle is essential to minimize errors that may arise during any stage of manufacturing, including development, production, and delivery.
This is a critical concept for product developers since identifying and addressing errors as early as possible can help reduce costs significantly.
Various studies have shown that there is a 1:10:100 cost/time ratio among the three manufacturing categories. This means that identifying and correcting a mistake during production is likely to cost ten times more, both in terms of time and money, then if it were caught during development.
Following the same ratio, an error will then cost 100 times more to fix if it actually reaches consumers. That’s why applying quality inspection at the end of the production line is a risky move that few major companies dare to take. Big, organized, and customer-oriented companies are now focusing on inspecting earlier to prevent catastrophic consequences and save resources.
Automated Quality Control as a Solution
In order to lower the risk of errors slipping through in all stages of the quality control process, businesses are turning to technology to increase the accuracy of their inspections. Automated quality control is a comprehensive solution that not only allows all differences and errors to be found in critical content, it also speeds up inspections saving businesses time and money.
Businesses can use automated quality control to conduct digital inspections on both hardcopy samples and digital files. This eliminates the need for manual proofreading. By using automated quality control, users can compare sample files to approved content automatically and detect any discrepancies between the two immediately.
The role of automated quality control consists of both speeding up the proofreading process and improving the accuracy of content. To learn more about automated quality control read our detailed blog post here.
How Automated Quality Control Gets Products to Market Faster
Automated quality control technology helps businesses get products to market faster without compromising quality. By automating manual tasks that would otherwise take hours to complete, this technology speeds up the entire quality control process and significantly cuts down proofreading time. In turn, errors can be detected quickly and accurately as inefficiencies like fatigue and human error are eliminated.
Some benefits of implementing automation technology in your business’ quality control process include:
Time benefits of automated quality control
Efficiency benefits of automated quality control
To find out more about how automated quality control speeds up product time to market, read our blog post.
Automated Quality Control for Increased Efficiency
Automated proofreading software simplifies workflow processes, leading to better, more efficient processes. In order to increase productivity in the workplace, practices need to be instilled that allow businesses to get tasks completed more quickly without compromising on the quality of the work.
The implementation of automated quality inspection will enable corporations to rely entirely on digital verification while almost completely reducing and eliminating the need for manual proofreading.
Automated proofreading eliminates the need for overly complicated processes and simplifies to only a couple of steps. Once content is created, the software inspects the entire file looking for discrepancies in the master document and the print.
Errors will be identified within seconds, with the software pinpointing all differences in the document. All that’s left is for quality control teams to make the necessary corrections, and the content is ready to be printed and published.
The software also eliminates the need for large teams to overlook proofreading processes and brings it down to just a few people and the software. Proofreading times are also heavily decreased as the software thoroughly inspects all aspects of the document finding errors that would be extremely difficult to find through manual inspections, such as those of colour, graphic, and barcode deviations.
Find out more about how you can increase efficiency with automated quality control here.
Industry Benefits of Automated Quality Control
Automated quality control presents many benefits for diverse industries operating within regulatory markets. It not only ensures stringent compliance with regulatory standards and guidelines it also efficiently detects deviations and errors, reducing the likelihood of non-compliance and associated penalties.
Some industries that can extremely benefit from automated quality control include:
Automated quality control benefits pharmaceutical companies by ensuring accuracy, reducing errors, and speeding up processes. It helps meet regulatory standards, brings products to market faster, and provides valuable data for continuous improvement.
Automated inspections for pharmaceuticals have become essential to the day-to-day affairs of companies wishing to attain the efficiency and error-free work that is demanded of them. In addition to the rigorous quality control implemented on the products themselves, it is important to ensure the compliance of packaging and labeling information.
If labels and packaging components are not up to the same quality standards as the products themselves, there could be detrimental consequences to both the brand and the consumer. In this article, we explore the numerous ways that automated packaging inspections can benefit the pharmaceutical industry.
Ensure the accuracy of your critical pharmaceutical content through automation.
The Importance of Quality Inspection in Print and Packaging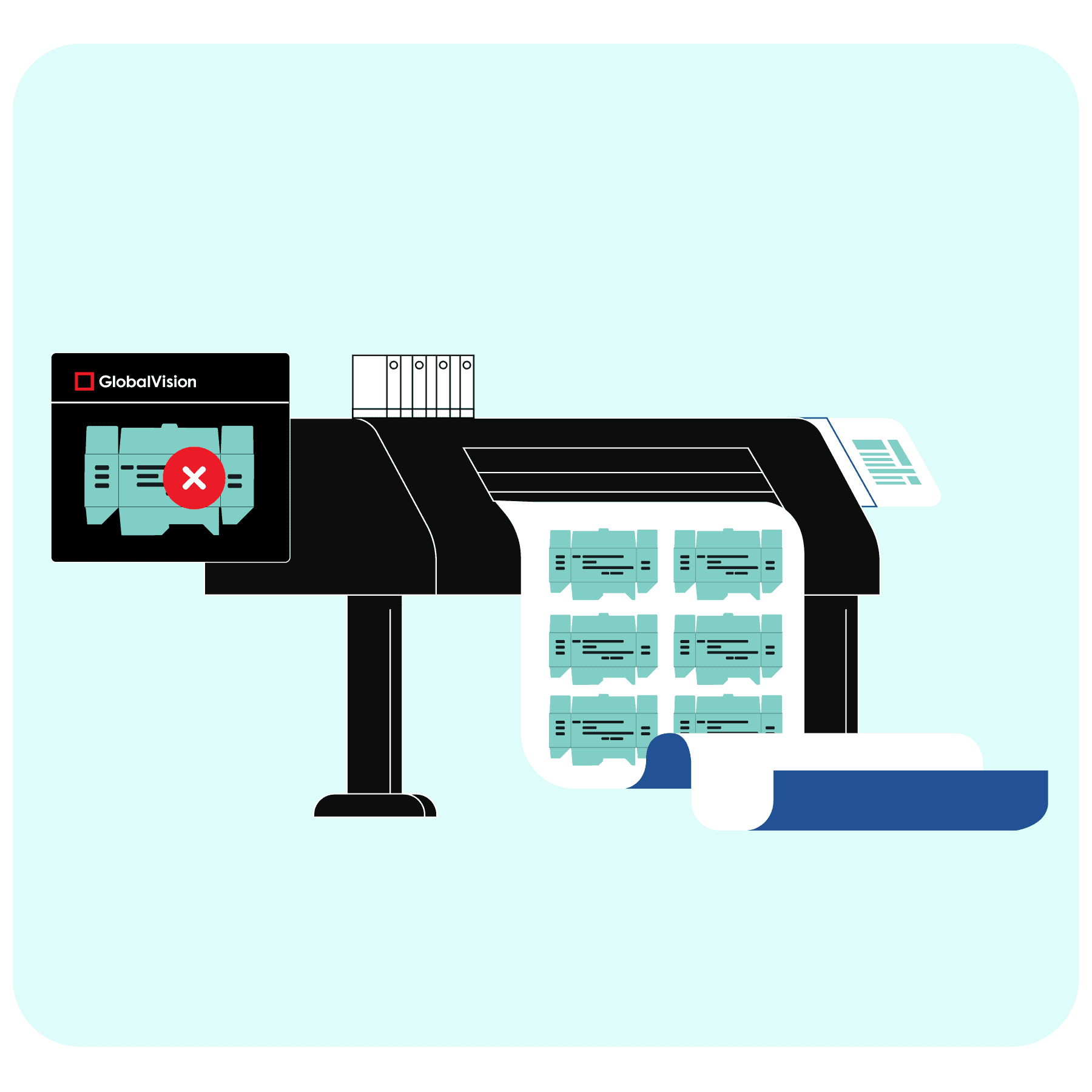
Companies needing to print in bulk will find that having a quality inspection process is extremely beneficial. Print inspection systems can provide the assurance and quality control your company needs to minimize mistakes. They will also guarantee the delivery of consistent results that will enhance your brand’s image through error reduction.
In fact, the role of quality inspection systems becomes even more vital when dealing with offset commercial printers. Given that most printing presses operate at incredibly high speeds, irregularities in the final product are more than possible, if not mandatory.
Quality inspection software in print and packaging – as opposed to manual inspection systems – provides the necessary accuracy to achieve consistent and flawless results on a regular basis.
Modern Solution for Prepress
Modern print inspection systems consist of advanced technology that links with your printing press or web rewinder to achieve exceptional results. It works by integrating vision systems (cameras), web viewers, and high-tech software that will catch any errors in time before they are printed in bulk.
Over the years, these quality inspection systems have been developing at a rapid pace. Nowadays, you can find multiple options online at very competitive prices. In fact, given that they require little to no maintenance, one of the key advantages of implementing these non-traditional tools is that you only need to make a one-time investment. Other benefits include ease of use and operation and the ability to have more control over your result.
Benefits of Automation for Creative Agencies
Quality control systems are essential to ensuring that no content errors affect the quality of campaigns and other projects.
Built to ensure the quality of work at all stages of the agency workflow, GlobalVision’s software eliminates inefficient and costly errors that can delay projects and negatively impact client satisfaction. Manual proofreading simply isn’t accurate enough to meet the defined standards that clients expect from their hired agencies, especially when multiple departments are involved.
The various divisions of a creative agency require standardization when it comes to quality control, which is where GlobalVision’s software comes in. From ensuring the accuracy of copies and vendor proofs to graphic and editorial consistency, automated quality control elevates your agency’s credibility and inspires confidence in your clients.
Everything you need to know about automated quality control for creative agencies is right here.
The Role of Quality Control in Consumer Goods
Automated quality control has emerged as a game-changer for the Consumer Packaged Goods (CPG) industry, revolutionizing the way manufacturers ensure product excellence. By leveraging advanced technologies like machine learning and computer vision, automated quality control streamlines and optimizes various aspects of production.
It enhances accuracy and consistency in inspecting content, identifying defects, and monitoring the manufacturing process.
With real-time data analysis, potential issues can be detected swiftly, reducing the risk of faulty products reaching consumers. This not only enhances overall product quality but also minimizes waste and recalls, resulting in cost savings for CPG companies.
Furthermore, automation eliminates the need for extensive manual inspections, freeing up human resources for more strategic tasks, such as research and development, fostering innovation, and driving long-term growth in the industry. Overall, automated quality control empowers the CPG sector to deliver higher-quality products, bolster consumer trust, and maintain a competitive edge in a dynamic market landscape.
Automated Proofreading to Replace Obsolete Difference Checker Tools
According to official Food and Drug Administration numbers, the majority of product recalls are caused by labeling errors. How can companies accurately keep track of the quality of millions of products being packaged daily? It’s definitely a challenge, but that’s why proofreading is crucial in order to release products into the market with complete confidence.
Over the last 20 years, companies have come to realize that human inspection is inherently prone to error. There’s no arguing that, as safety regulations are continuously increasing, automated inspection has become the gold standard for the industry.
Early on, ”diff checker” and ”text compare” tools were the ultimate proofreading solution in the market, allowing for the comparison of characters between two documents and spotting changes in the text before they reach the printer to ensure data integrity.
With the advent of sophisticated machine vision systems and automated proofreading software, technology is progressively becoming more powerful, efficient, and easier to set up and use.
Modern text inspection tools can even generate progress reports, outlining all errors corrected for faster approval times. Meanwhile, plain old document comparison software continues to fall behind when it comes to innovation.
Read more about how automated proofreading is replacing obsolete difference checker tools.
Automate Your Workflows for Success
Quality inspection is an essential part of every production line.
Those who do not understand how valuable they are, are not looking at the big picture. Money-wise, the cost of manufacturing a product extends far beyond the build cost, as it continues across its lifecycle, from support and delivery to warranty claims.
The one-time investment in quality inspection software can reduce future costs relating to customer support, warranty returns, and rejected or returned items. They can even add value to your company, as you’ll count on a competitive defense tool that will eventually pour more money into your pockets.
Ready to step into the world of automated quality control? Request a demo of our innovative proofreading software and see how this technology can revolutionize your everyday business practices.
Transform your quality control processes with GlobalVision’s cloud-based quality inspection tool.
Upgrading Your Brand? Streamline Brand Transitions With Document Comparison Software
Date: August, 2023 | Category: Quality | Author: Hana Trokic
In the world of design and communication, font plays a pivotal role in conveying the right message and aesthetics.
Microsoft took note of this and recently made the significant decision to transform its brand through a typeface upgrade. They said goodbye to their old trusted friend, Calibri, and introduced a new default typeface, Bierstadt – now called Aptos. While this transition enhances and upgrades the visual appeal of the Microsoft brand, it also raises concerns about content errors that might arise due to the new typeface changes.
Making big brand changes like these is never easy and as Microsoft notes, it’s quite intimidating changing a typeface that was used for 15 years. The fonts we saw on our screen for over a decade not only became a part of the brand’s visual identity but a part of the brand itself.
A long and strenuous task, upgrading your brand is a complete journey but document comparison software is a solution that makes it that much easier. Not only does it prevent content errors, but it also ensures a seamless transition to new typefaces by automating a good deal of the proofreading process.
The Importance of Upgrading Your Typeface
Microsoft’s decision to transition to the Aptos font comes with a range of motivations. A refreshed font will give the company a more contemporary and uniform visual identity, aligning with modern design trends.
Also, technology has changed significantly in the last 15 years, and the screens and computers we use today are far from what they used to be. This posed the need for a new font that was better suited to high-resolution screens and was sharp, modern, and an overall better fit for the sleek machines we use today.
However, font and brand transitions can pose millions of challenges, particularly in maintaining content quality and accuracy.
The shift in font styles can inadvertently introduce formatting discrepancies, typographical errors, and inconsistencies that could affect the overall user experience. This is where document comparison software steps in to maintain content integrity.
The Role of Document Comparision Software
Document comparison software, like GlobalVision, offers an advanced solution to ensure the smooth implementation of font transitions while preserving content quality.
Here are some key ways in which it can help:
Error Detection: Document comparison software is designed to identify various types of errors, including text, spelling, image, graphics, color, print, and more. Regarding font changes – spelling mistakes, grammar errors, punctuation errors, and formatting inconsistencies are more common issues. These errors can easily go unnoticed due to letter shape and size differences. Document comparison software’s algorithms can detect these errors regardless of font style, making it an indispensable tool in maintaining content accuracy.
Consistency Check: Maintaining consistent terminology and style is crucial during font transitions. Document comparison software detects errors and ensures that your content adheres to specific guidelines and style preferences. This is particularly important for brands that want to uphold their image consistently across various communication materials.
Efficiency: Document comparison software significantly accelerates the review process. Manually combing through content to identify errors and inconsistencies can be time-consuming and prone to human oversight. Document comparison software automates this process, allowing your team to focus on more creative and strategic tasks during your brand’s upgrade.
Adaptability: Document comparison software adapts effortlessly in the case of a font transition. It recognizes that the font has changed and evaluates the content based on the new typography, reducing the likelihood of overlooking errors due to the font switch.
Seamless Brand Transitions with Document Comparison Software
GlobalVision, a leading document comparison software, offers a comprehensive solution for businesses undergoing font transitions like Microsoft’s shift to Aptos.
Here’s how this innovation in quality inspection software it can make the process seamless:
Comprehensive Content Inspections: GlobalVision’s advanced algorithms run comprehensive checks of files and documents. This allows the software to identify any and all errors across critical content which include various fonts. This also ensures that content remains error-free even after a typeface change.
Customizable Inspections: The software allows users to set specific guidelines and rules for content quality inspections, ensuring consistency and adherence to brand standards throughout the transition.
High Volume Inspections: GlobalVision enables batch processing, making reviewing large volumes of content effortless during any transition period. A crucial feature as seemingly infinite amounts of content will need to be proofread to ensure new changes are updates have been made.
Effortless Collaboration: The software supports collaboration among team members, allowing them to review and approve content changes collaboratively, thus expediting the entire process.
Ensure Your Brands Integrity
Typeface or font transitions are necessary and exciting aspects of brand and design evolution, but they must be approached with caution to maintain content quality, accuracy, and, ultimately your brand’s image.
While it’s a heavy and time-consuming task, upgrading your brand’s image is necessary to maintain relevance in the market and stay competitive. Document comparison software can play a vital role in this process, offering a reliable solution to detect errors, ensure consistency, and make seamless brand guideline transitions.
As companies, designers, and brands embrace changes to refresh their visual identities, the integration of a comprehensive solution such as document comparison software becomes crucial to uphold content integrity in the midst of change.
Looking to guarantee the consistency and accuracy of your brand? Take the first steps towards flawless content and accelerated time-to-market using GlobalVision’s state-of-the-art document comparison software. Begin experiencing the advantages of our cutting-edge software today.
Exploring Effective Security Measures: Safeguarding Data and Building Trust
Date: August, 2023 | Category: Compliance | Author: Hana Trokic
In today’s digital age, information security has become an utmost concern for organizations and their customers. As cyber threats continue to evolve, businesses must prioritize safeguarding sensitive data and ensuring its confidentiality, integrity, and availability.
ISO 27001 is one of the leading international standards for information security and provides a comprehensive framework to achieve these goals.
What is ISO 27001
ISO 27001 outlines the necessary requirements for an information security management system to follow. This comprehensive standard offers valuable guidance to businesses on establishing, implementing, maintaining, and continually enhancing their information security management system.
Achieving compliance with this framework signifies that an organization has implemented a system to effectively manage risks associated with the security of their data, including that of their customers. This system aligns with the best practices and principles outlined in the International Standard, ensuring a systematic and cost-effective approach to safeguarding sensitive information.
ISO 27001 consists of 3 main pillars:
The Benefits of ISO 27001 Certification
There are many benefits of being certified. They include:
Instill Trust for Our Customers & Prospects: Demonstrate our commitment to maintaining the highest level of security for our customer’s and prospects’ information, which builds trust and confidence in our ability to protect their data.
Improve Security & Protection of All Data: Ensure the confidentiality, integrity, and availability of both sensitive and non-sensitive data, reducing the risk of data breaches and other security incidents. This leads to a more secure and protected environment, which is critical in today’s interconnected digital world.
Compliance with Regulatory Requirements: Our customers in regulated industries are required to comply with various regulatory requirements related to information security. This framework helps us demonstrate compliance with regulations as their supplier, providing them with confidence that their data is being accordingly.
Reduced Risk of Data Breaches: Data breaches can lead to significant financial and reputational losses for both us and our customers. ISO 27001 provides customers with peace of mind knowing that their data is less likely to be compromised.
Improved Business Continuity: The framework requires us to have a business continuity plan in place, which helps to ensure that critical business functions can continue in the event of a disruption or disaster. This helps customers maintain the continuity of their critical business activities and reduces the risk of disruptions.
Better Communication: ISO 27001 requires us to communicate our information security policies and procedures to all relevant stakeholders, including customers. This helps us improve communication between us and our customers, further promoting transparency and trust.
A Trustworthy Partner
Always adhering to the most recent stringent measures, GlobalVision has always been committed to the protection and integrity of information to ensure that data remains protected for our users, employees, and third parties.
With validated systems, comprehensive internal audits, and multiple verifications during the development and delivery stage, we address data integrity issues and can be counted on by all regulated companies.
This certification provides GlobalVision with yet another affirmation that proves this to be true. For highly regulated companies in the pharmaceutical, medical device, biotechnology, and financial services industries, ISO 27001 certification becomes a crucial factor when considering potential partners or suppliers. GlobalVision’s commitment to security strengthens our position as a market leader for continuing innovation with trust.
More Than Just a Standard
ISO 27001 is more than just a standard; it is also a powerful tool that helps organizations protect their information and build trust with their customers and prospects. By adhering to its three pillars of confidentiality, integrity, and availability, organizations can ensure robust information security, reduced risk of breaches, and enhanced business continuity.
For GlobalVision, this certification is yet another attestation to its commitment to keeping customers first. Whatever their needs be, we strive to go above and beyond to offer the best and more secure services and products to our users.
Embracing this framework not only strengthens information security but also reinforces our commitment to safeguarding the data entrusted in our care.
To learn more about GlobalVision, please visit our webpage here.
IPG Health Increases Efficiency in Document Reviews With GlobalVision
Date: August, 2023 | Category: Customers | Author: Hana Trokic
About IPG Health
IPG Health is a network of world-renowned agencies focused on health communication and marketing. Collectively, the network is made up of over 45 agencies that are spread across six continents and 6,500+ employees driven by a company-wide obsession with harnessing creativity, technology, science, and data to inspire behaviors that fuel better health.
IPG Health’s clients include the top 20 global pharmaceutical companies as well as countless startups, biotech companies, biopharma companies, and a variety of life science companies. In everything they do, IPG Health is relentlessly focused on doing what’s right for their clients, their brands, and their people.
The Challenge: Keeping Content Accurate While Facing Regulatory and Time Constraints
Due to the fast-paced, highly regulated, and critical nature of their field of work, IPG Health must focus significant time and resources on ensuring the accuracy of every piece of content and creative material produced.
Kyle Richards, EVP, Executive Director, Editorial, at IPG Health, gave us a detailed look into some of the key challenges his teams and department face in the project development process. He noted that their main goal was to deliver accurate content for their clients and a large part of ensuring that was through accurate and fast proofreading processes.
IPG Health deals with large volumes of medical and scientific materials that are often lengthy and technical, and that include special characters that further complicate proofreading. When reviewing the documents manually, the proofreading process can be very time-consuming. Traditionally, this often led to the need for additional proofreaders at the late stages of the project development process to avoid bottlenecks while ensuring accuracy.
Further, duplicate proofreading reviews at key project milestones were part of their standard operating procedure to ensure accuracy as projects moved through the late-stage production steps. The need for a second proofreader so routinely added a resource management challenge to the quality control process.
“We found that there were bottlenecks with regard to turning projects around and getting them back to our clients. We were coming up against these crunch times to turn around content quickly for product launches, and the manual proofreading process was slowing us down.”
The Solution: Increase Content Review Efficiency and Maintain Accuracy Through Automation
When IPG Health first sampled GlobalVision’s automated solutions 10 years ago, they instantly noticed the incredibly accurate results it was producing proofreading files. On top of that, even at a time before the routine use of digital tools, GlobalVision products were easy enough to use to enable a broad rollout within the editorial teams.
For over a decade, this accuracy has proven reliable, as IPG Health still trusts GlobalVision as an important component of its editorial process.
“A lightbulb went off when we were proofreading a 35-page product package label. The manual proofreading took most of the day to complete, yet when I ran the files through [GlobalVision], it took all of 30 minutes. It caught all the errors the manual proofreader found plus two additional errors that would have otherwise slipped through.”
In many cases, IPG Health teams work with pre-approved client content that needs to be included in their materials. While there is plenty of room for their renowned creativity in other areas, in these cases they must strictly adhere to the approved language. To help with this, GlobalVision is used to ensure complete accuracy, in addition to its use for late-stage proofreading to ensure content integrity.
Kyle Richards notes that due to their text-heavy content, the Text Inspection tool is the feature they rely on most. They also benefit greatly from the built-in Spell Check and Custom Dictionaries, which proved to be extremely helpful when proofreading health-related material. Graphics inspection is also used frequently, as diagrams, charts, and visuals are commonly included in the content.
Throughout the years, IPG Health found numerous advantages of using GlobalVision’s file comparison technology but has particularly noted three main software benefits:
Automated inspections drastically decrease the time needed to proofread lengthy, technical, health-related content. In general, it decreases proofreading times at IPG Health by about 75%-80%.
It hasn’t been just IPG Health reaping the benefits of GlobalVision, their clients have also received the benefits. Kyle Richards notes that “Clients are always looking to be more efficient with their budget. With GlobalVision, we’re able to do more work within the same time, meaning they’re getting more for their dollar.”
“Clients are always looking to be more efficient with their budget. With GlobalVision, we’re able to do more work within the same time, meaning they’re getting more for their dollar.”
As they are continually working to improve the efficiency of their quality control processes, IPG Health recently began implementing GlobalVision’s newest innovation in cloud-based proofreading software, Verify. During the switch to the latest software, the editorial teams noted that it was very easy and intuitive to use and that it has made proofreading even easier than before.
For a decade and counting, IPG Health has had nothing but praise for GlobalVision and the advantages it has added to its quality control workflow. When asked if he would recommend the software to others considering automated proofreading, Kyle Richards quickly answered, “Yes, absolutely. From an editorial perspective, it supports our proofreading efforts and allows us to do a challenging aspect of our role much more efficiently, which also buys us more time to tackle other aspects of our role that bring higher value to the team.”
Take the first step toward error-free content with GlobalVision’s automated solutions. Learn how proofreading software can help your quality control workflow, and start reaping the benefits today.
Experience PACK EXPO Las Vegas 2023 with GlobalVision!
Date: August, 2023 | Category: Company | Author: Hana Trokic
GlobalVision is thrilled to announce we are attending PACK EXPO Las Vegas, the premier event for the packaging and processing industry, as a proud sponsor.
Join us from September 11 – 13th, 2023, as we connect with industry leaders and professionals, showcasing our cutting-edge proofreading solutions and networking with like-minded individuals in the industry. Also, be sure to visit our booth live at the event for insights into our products and exclusive offers, including a complimentary Artwork Creation Guide and a chance to win a $25 Amazon Gift Card!
What to Expect at PACK EXPO Las Vegas 2023
Biennially, PACK EXPO Las Vegas unites suppliers of packaging and processing solutions with end users in over 40 diverse industries. This dynamic event serves as a platform for exhibitors to introduce new products, demonstrate their industry innovations, and collaborate with customers to address their specific needs.
With an extensive array of packaging and processing technologies being showcased, attendees have the opportunity to explore thousands of options and find transformative solutions to overcome their packaging and processing challenges.
Meet The GlobalVision Team and Learn About Our Latest Innovations
At PACK EXPO Las Vegas 2023, GlobalVision is set to present our state-of-the-art automated quality control solutions, specially designed to address content efficiency challenges in regulated industry. With our file inspection technology leading the way as an enterprise solution, we are eager to engage professionals in the packaging industry and beyond to demonstrate how our platform can streamline their document comparison processes.
Visit our Booth
Look for us at booth #N-9809 to not only learn about our products and enterprise solutions but, to also take part in some special surprises we have prepared for attendees! At our booth, you will have the opportunity to:
Don’t miss out on these opportunities and many more. Make sure to check us out in person by registering for PACK EXPO Las Vegas! Click here to register now.
Make the Most of PACK EXPO Las Vegas 2023
GlobalVision has been helping businesses bring confidence to quality control for over three decades and counting. Our mission is to build software that standardizes quality approvals for regulated industries while eliminating errors in critical content and automating inspections.
If you would like to learn more about our products and what we do, be sure to contact us for more information. If you are attending PACK EXPO Las Vegas 2023, visit our booth and say hello to the team!
We can’t wait to meet you and share the latest advancements in our solutions for the packaging and processing industry. Mark your calendars for September 11th to 13th, 2023, and prepare for an amazing experience at PACK EXPO Las Vegas. We’re eager to see you all there!
Accelerating Success: GlobalVision Revolutionizes iNova Pharmaceutical’s Product Launches with Unmatched Accuracy and Speed
Date: August, 2023 | Category: Customers | Author: Hana Trokic
About iNova Pharmaceuticals
iNova Pharmaceuticals is a global organization dedicated to the development, marketing, and distribution of a diverse range of prescription medications and consumer health products. Operating across Asia, Australia, New Zealand, and Africa, iNova serves over 20 countries internationally.
With a team of 550 professionals spanning three continents, their goal is to provide trusted products that improve people’s health and well-being. Their diverse product portfolio includes weight management, cough and cold remedies, health supplements, dermatology, sun care, and female health products. Their products have established a legacy of over 50 years, earning a prominent place in countless family medicine cabinets.
iNova Pharmaceuticals is dedicated to delivering effective and high-quality pharmaceuticals manufactured in accordance with the most stringent international standards.
The Challenge: Overcome Inefficient Outsourcing and Complicated Revision Workflows
In late 2017, quality teams at iNova Pharmaceuticals underwent organizational changes that allowed them to bring more control into their quality assurance processes, eliminating the need for outsourced third parties and complicated revision workflows.
Artwork management in particular was a main area of focus. At the time, teams at iNova were working with an external studio that managed most of their artwork processes. While the studio provided a tool that allowed iNova to complete product or artwork reviews online, the tool was not managed by them directly, giving them little control over the process.
To resolve this issue, Phil Sami, Group Operations Improvement Director was given the task to create an artwork process and workflow that was consistent and streamlined across all three regions of the organization.
What he soon discovered was GlobalVision’s innovative proofreading software. The implementation of the system in early 2018 would allow them to bring back ownership of their revision and quality assurance processes internally and allow them to have total control of all their artwork pieces as well as changes and revisions.
“GlobalVision is a great software to compare the print proofs and perform quality checks of the artworks.”
The Solution: Take Ownership of Quality Reviews and Ensure Seamless Product Launches with GlobalVision
Through GlobalVision’s proofreading software, quality teams at iNova Pharmaceuticals were able to ease collaboration and once again take back ownership of their artwork management workflows. This implementation allowed them to ensure first-hand the accuracy of their files which ultimately led to faster and higher quality revisions as well as increase their overall speed-to-market for new product launches.
Phil Sami notes that besides the additional capabilities and features that allowed the company to enhance their revision workflows while ensuring compliance, a key factor in choosing GlobalVision was that the software was FDA-approved and it was difficult to find alternatives that offered compliance packages required by pharmaceutical industry software providers. GlobalVision was the comprehensive solution for their everyday quality assurance needs and stood out as the market leader.
“GlobalVision has been vital in artwork development as it helps pick up the tiniest of errors and differences both within text and graphics. It also saves so much time by not having to do manual proofreading.”
He adds that a majority of the users are in the company’s Quality department and that the software is primarily used in the following manner:
“GlobalVision has been tremendously useful in decoding barcode values hence ensuring we deliver our products with GS1-compliant barcodes to our customers”
Another crucial feature for iNova Pharmaceuticals is the software’s ability to select specific areas of a file that need to be inspected, namely, the Zoning Tool. GlobalVision allows users to pinpoint exact areas of a file, whether text or graphics, allowing for more detailed and precise inspections.
Phil adds that this feature is particularly beneficial when working on files in foreign languages. As iNova distributes products to markets globally, quality teams must work on files in languages and alphabets that are not native to them. The Zoning Tool allows for granular inspections of foreign content and more accurate visual inspections of graphic components within seconds.
Conclusion: iNova Pharmaceutical Revolutionizes Revision Workflows
Since 2018, quality and artwork teams at iNova have been reaping the many benefits of GlobalVision and their revision processes have become unimaginable without the market-leading proofreading software. As they are consistently reviewing and improving their quality control processes, iNova Pharmaceuticals is in the process of implementing GlobalVision’s newest innovation in cloud-based proofreading software, Verify, to further drive speed and accuracy in their proofreading processes.
As Verify is integrated with Veeva, another system used by iNova Pharmaceuticals, teams predict that this implementation will save additional time, approximately 2-3 min per piece of artwork, as switching between the different platforms will no longer be needed.
In the future, iNova Pharmaceuticals plans to continue to work alongside GlobalVision, implementing and updating its software and workflows to ensure the highest quality products they have become known for, all the while guaranteeing customer satisfaction and loyalty for many more years to come.
Take the first step towards error-free content and quicker time-to-market with GlobalVision’s automated proofreading software. Start reaping the benefits of automated quality control today.