Date: April, 2016 | Category: Compliance | Author: Reuben Malz
No matter what industry you are in there are always new regulations that impact the way you create labeling artwork. Staying compliant with regulatory labeling obligations is a complex undertaking that if done wrong may result in undesired errors or reworking.
Regulatory Label Compliance with GlobalVision
GlobalVision provides a Quality Control Platform to proofread artwork, text, print, barcode, and Braille and is designed to help you maintain regulatory compliance.
Major regulations now in place or planned are listed below along with an appropriate GlobalVision solution that can enhance the quality and speed of inspections:
Chemical
OSHA – Hazard Communication Standard (United States)
In order to ensure chemical safety in the workplace, information about the identities and hazards of the chemicals must be available to workers.
WHMIS 2015 – Workplace Hazardous Materials Information System (Canada)
A regulation to provide health and safety information on hazardous products (labeling, safety data sheets) that is intended for use, handling, or storage in Canadian workplaces.
New Requirements
Chemical manufacturers and importers will be required to provide a label that includes a harmonized signal word, pictogram, and hazard statement for each hazard class and category. Precautionary statements must also be provided.
The GlobalVision Solution
GlobalVision Text Inspection Solution
- Validates that the correct font and text size are used on the label for artwork.
- Verifies that the correct font, size, and style are used to differentiate the Signal word
GlobalVision Graphics and Artwork Inspection Solution
- Verifies artwork including pictograms on a pixel-by-pixel basis.
Food
Food Labeling Modernization Act (Canada)
Update of food packaging labels designed to improve access to information and to increase awareness, to help consumers to make informed decisions about the food they buy.
EU Regulation 1169/2011 (European Union)
Revision of product information on product packaging and online stores pertaining to food and beverages sold in the EU. The objective of which is to standardize food labeling and provide greater clarity to consumers concerning ingredients, nutrition and allergens.
FDA Nutrition Fact Labels Revision (United States)
Update of food labels in the United States to reflect up-to-date serving sizes, as well as percent daily value, designed to better inform consumers.
ACC Country of Origin Labels (Australia)
Revision of food labels to include the addition of a statement and a bar graph indicating the proportion of Australian ingredients by weight.
New Requirements
New food labeling directives all specify lists of mandatory information and details on how the label information should be formatted and displayed – including minimum font requirements, new artwork, and tables on food packaging included in the new formats.
The GlobalVision Solution
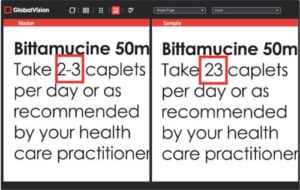
GlobalVision Text Inspection Solution
- Validates that the correct font and font size are used during typesetting. The font type and size have a direct correlation to the X-Height of the final printed package.
- Verifies that the correct font, size, and style were used to define different elements.
GlobalVision Graphics and Artwork Inspection Solution
- Compares previous artwork to new version artwork, to ensure stylistic differences are present.
GlobalVision Print Inspection Solution
- Inspects X-Height in mm on the final printed packaging.
- Compares the previous package to the new version of the package, to ensure stylistic differences are present.
GlobalVision Barcode inspection
- Verifies and grades the barcode to ensure accuracy and scanability.
Braille
ISO 17351:2013 (European Union)
Standard outlining the requirements and providing guidance for the application of Braille to the labeling of medicinal products.
Safety and Innovation Act (Pub. L. 112-144, 126 Stat. 993) (United States)
A series of best practices on how to offer guidance to pharmacies and provide accessible prescription drug container labels to patients with visual impairments to enable them to manage their medications independently and privately.
New Requirements
New Braille regulations require Braille on the outside packaging of all medication – including name, and dosage of medication.
The GlobalVision Solution
GlobalVision Braille Inspection Solution
- Ensures that Braille Dot Height meets the 0.20 mm requirements ensuring readability by visually impaired persons.
- Verifies the Braille character spacing
- Translates Braille into readable text
Pharmaceutical

Directive 2001/83/EC Article 11 (European Union)
Updated packaging and labeling regulations for the EU
FDA Opioid Action Plan (United States)
Revised labeling requirements is to better inform doctors about the risks of opioids and how to prescribe these drugs safely.
FDA NSAID Warning (United States)
Revised warning labels that non-aspirin non-steroidal anti-inflammatory drugs (NSAIDs) may cause heart attacks or strokes
New Requirements
Packaging content must match the approved QRD template from the European Medicines Agency (EMA) (for EU).
Multiple modifications and additions to the packaging, labeling, warning labels, and associated literature.
The GlobalVision Solution
GlobalVision Text Inspection Solution
- Ensures that the approved content contained within the QRD template is consistent with the carton, folding boxes for medicinal products (for EU)
- Medical spell-check
- Complex table handling
- Detect deviations between packaging, labels, leaflets, and the text of the Annexes
GlobalVision Graphics and Artwork Inspection Solution
- Compares previous label artwork to new version artwork, to ensure stylistic differences are present.
For more information on the GlobalVision Quality Control Platform
and how it can help with your compliance requirements please visit globalvision.co
Our helpful guide walks you through the major steps of a typical package printing process, and how you can ensure a flawless delivery, every time.

Canadian Health Product Labeling – The Product Facts Table
Date: July, 2016 | Category: Proofreading | Author: Reuben Malz
Label content pertaining to non-prescription healthcare products is of great concern to the government of Canada.
This not only includes what appears on the label, but also the wording, how it appears, and how easy it is to understand are all of vital concern.
Canadian Health Product Labelling
What are non-prescription healthcare products?
Non-prescription healthcare products are defined in the Food and Drugs Act as including any substance or mixture of substances manufactured, sold, or represented for use in the prevention, treatment, symptomatic relief, cure, or risk reduction of diseases, injuries or chronic conditions that individuals can recognize and manage on their behalf, either separately or in participation with professionals.
Through Health Canada’s Plain Language Labelling Initiative, new regulations have recently been introduced to help consumers choose the right product as well as how to use it safely. Included within these new regulations are the requirements for a Facts Table on the outer label of non-prescription drugs.
The Facts Table will be mandatory for all new products beginning in June 2017. For all existing non-prescription products, the Facts Table will be mandatory by June 30th, 2021.
The new table requirements will apply to thousands of non-prescription drug products sold throughout Canada, and will be called the “Drug Facts Table.” The new Drug Facts Table uses simple language and an easy-to-read format. It is modeled after Canada’s Nutrition Facts table on foods as well as on the U.S. Drug Facts box used for non-prescription drugs.
*Update*
Since the publishing of this article, a new Plain Language Labelling Regulations for Prescription Drugs has been put into effect as of the 2020/04/01.
The Drug Facts Table is Designed to Help Consumers
The drug facts table helps consumers through the following:
For Canadian manufacturers and companies distributing non-prescription drugs into Canada, this new labeling regulation will require companies in less than one year to plan and execute updates to hundreds of SKUs to remain compliant.
GlobalVision has been the leading provider of quality control solutions to help pharmaceutical companies and their print suppliers maintain compliance throughout the production workflow.
Learn how GlobalVision has helped top Pharmaceutical Companies in quality control.
For more information on GlobalVision’s Quality Control Platform, please visit globalvision.co.
How do You Inspect Packaging of Bottles, Cans and Cylinders
Date: April, 2016 | Category: Proofreading | Author: Reuben Malz
Packaging and labeling are driven by a number of factors. First, you have the ergonomics of the package, ensuring that the object is packaged properly ensuring protection, convenience, and the proper message is communicated.
Another key factor is its market appeal, the artwork, colors, and fonts that companies use directly influence sales. There is truly a science behind it. However, it is important to ensure that there are no errors because no matter how nice the package is, consumers will not buy goods that are mislabeled.
An error in labeling is the number one reason why products are recalled. Companies have paid millions of dollars in fines and penalties to recall products for simple product mislabeling; in fact, the average cost of a product recall is between 5 – 10 million dollars. Finally, the damage to a company’s reputation can be immeasurable and could amount to a company failing.
Inspecting Labels on Bottles, Cans, and Cylinders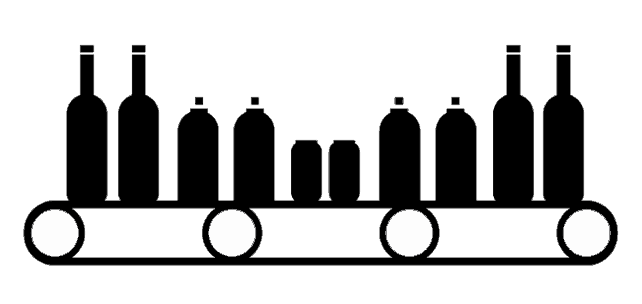
To ensure the package is error-free, progressive companies now use automated proofreading solutions throughout the design and printing of the package and label. In fact, for every phase of the packaging workflow, a different proofreading solution can be used for inspection purposes anywhere from text and artwork inspection to barcode and braille verification.
To inspect a printer proof versus the approved artwork is a relatively straightforward job and done by most printers before completing the entire press- run. A sample of the printed piece is first scanned with a Roll or Flatbed scanner and then uploaded into a print inspection solution like the print inspection tool by GlobalVision. Packaging that can be laid flat, such as labels and folding cartons, poses little problem. However, until recently, packaging that is cylindrical poses a unique challenge to printers and their customers.
In the past, companies would cut cylindrical containers such as cans and tubes, try to get it flat as possible, and lay them on a flatbed scanner. This posed a few critical problems, the first being that you can never really get the sample container flat enough. Secondly, it is very time-consuming and can be a little dangerous. Also, you can severely damage or at a minimum shorten the scanner’s life.
Greater Inspection Accuracy with GlobalVision:
GlobalVision’s innovative automated proofreading software has been the leading developer of cylindrical inspection solutions for over two decades. The comprehensive quality control solution has the ability to inspect packaging and labels of the following:
With so many regulations affecting pharmaceutical and CPG companies, the need to ensure packaging accuracy has never been greater. With GlobalVision, even printed cylindrical items can be effectively and rapidly inspected, safeguarding against errors, reprints product recalls, and the accompanying financial loss.
If you want to begin streamlining your label and packaging inspection processes, request a free trial today for GlobalVision’s Print Inspection Solutions
Also, learn more about the pressing need to scan cylinder packaging.
Having to Rework Label Artwork Again and Again to Stay in Compliance?
Date: April, 2016 | Category: Compliance | Author: Reuben Malz
No matter what industry you are in there are always new regulations that impact the way you create labeling artwork. Staying compliant with regulatory labeling obligations is a complex undertaking that if done wrong may result in undesired errors or reworking.
Regulatory Label Compliance with GlobalVision
GlobalVision provides a Quality Control Platform to proofread artwork, text, print, barcode, and Braille and is designed to help you maintain regulatory compliance.
Major regulations now in place or planned are listed below along with an appropriate GlobalVision solution that can enhance the quality and speed of inspections:
Chemical
OSHA – Hazard Communication Standard (United States)
In order to ensure chemical safety in the workplace, information about the identities and hazards of the chemicals must be available to workers.
WHMIS 2015 – Workplace Hazardous Materials Information System (Canada)
A regulation to provide health and safety information on hazardous products (labeling, safety data sheets) that is intended for use, handling, or storage in Canadian workplaces.
New Requirements
Chemical manufacturers and importers will be required to provide a label that includes a harmonized signal word, pictogram, and hazard statement for each hazard class and category. Precautionary statements must also be provided.
The GlobalVision Solution
GlobalVision Text Inspection Solution
GlobalVision Graphics and Artwork Inspection Solution
Food
Food Labeling Modernization Act (Canada)
Update of food packaging labels designed to improve access to information and to increase awareness, to help consumers to make informed decisions about the food they buy.
EU Regulation 1169/2011 (European Union)
Revision of product information on product packaging and online stores pertaining to food and beverages sold in the EU. The objective of which is to standardize food labeling and provide greater clarity to consumers concerning ingredients, nutrition and allergens.
FDA Nutrition Fact Labels Revision (United States)
Update of food labels in the United States to reflect up-to-date serving sizes, as well as percent daily value, designed to better inform consumers.
ACC Country of Origin Labels (Australia)
Revision of food labels to include the addition of a statement and a bar graph indicating the proportion of Australian ingredients by weight.
New Requirements
New food labeling directives all specify lists of mandatory information and details on how the label information should be formatted and displayed – including minimum font requirements, new artwork, and tables on food packaging included in the new formats.
The GlobalVision Solution
GlobalVision Text Inspection Solution
GlobalVision Graphics and Artwork Inspection Solution
GlobalVision Print Inspection Solution
GlobalVision Barcode inspection
Braille
ISO 17351:2013 (European Union)
Standard outlining the requirements and providing guidance for the application of Braille to the labeling of medicinal products.
Safety and Innovation Act (Pub. L. 112-144, 126 Stat. 993) (United States)
A series of best practices on how to offer guidance to pharmacies and provide accessible prescription drug container labels to patients with visual impairments to enable them to manage their medications independently and privately.
New Requirements
New Braille regulations require Braille on the outside packaging of all medication – including name, and dosage of medication.
The GlobalVision Solution
GlobalVision Braille Inspection Solution
Pharmaceutical
Directive 2001/83/EC Article 11 (European Union)
Updated packaging and labeling regulations for the EU
FDA Opioid Action Plan (United States)
Revised labeling requirements is to better inform doctors about the risks of opioids and how to prescribe these drugs safely.
FDA NSAID Warning (United States)
Revised warning labels that non-aspirin non-steroidal anti-inflammatory drugs (NSAIDs) may cause heart attacks or strokes
New Requirements
Packaging content must match the approved QRD template from the European Medicines Agency (EMA) (for EU).
Multiple modifications and additions to the packaging, labeling, warning labels, and associated literature.
The GlobalVision Solution
GlobalVision Text Inspection Solution
GlobalVision Graphics and Artwork Inspection Solution
For more information on the GlobalVision Quality Control Platform
and how it can help with your compliance requirements please visit globalvision.co
Journey of the Package Printing Process
Our helpful guide walks you through the major steps of a typical package printing process, and how you can ensure a flawless delivery, every time.
Ensuring Barcode Accuracy from Packaging to the Patient
Date: April, 2016 | Category: Proofreading | Author: Reuben Malz
Barcodes have changed very little over the last 60 years. For pharmaceutical companies, the barcode has taken on a vital role throughout the product’s lifecycle from original packaging to in-hospital unit dose identification.
For pharmaceutical companies, a barcode helps to assure the origins of the drugs, which in turn helps in minimizing the possibility of genuine drugs being considered sub-standard or counterfeit. Many nations are also passing legislative initiatives making it mandatory for pharmaceutical companies to affix barcodes on all primary-level packaging.
For healthcare providers, the barcode on pharmaceutical packaging plays a critical role in the management of the patient. These barcodes can dramatically reduce the possibility of medication administration errors as well as give the provider an exact record of the patient’s medicinal intake.
Linear barcodes are the most common type of barcode used on pharmaceutical packaging. For medications, the linear barcode typically contains the 10-digit National Drug Code (NDC) number that identifies the manufacturer, product, and package size. However, a healthcare provider may also use its own unique barcode to point to a much larger descriptor of the item.
Barcode Packaging: Beyond What the Eye Can See
During the packaging workflow, the difference between a correct barcode versus an incorrect barcode is often too subtle to be noticed by the human eye. Even a barcode that appears correct may not be able to scan properly. The end result is that any error in the barcode may cost the pharmaceutical company millions of dollars in recalls as well as millions more in liability claims and loss of reputation.
Ensure Barcode Accuracy
At the healthcare provider level technology will make the barcode even more vital for the pharmaceutical industry. Healthcare providers are currently testing medication administration technologies (MATs) to ensure the patient’s safety by linking the barcode to the patient’s electronic health records. If this new technology is widely adopted, then pharmaceutical companies will most likely face stiffer penalties for not meeting barcode specifications on the packaging.
For over 25 years GlobalVision has been the industry leader in barcode quality control technologies, their knowledge of the pharmaceutical workflow process has given them unique insight into the critical role of the barcode as a packaging element.
Barproof automatically locates all barcodes on a label, carton, or press sheet and grades each according to CEN/ANSI/ISO standards and even inspects and decodes QR, linear, 1D, and 2D barcodes.
BarProof is also available as an app with the Quality Control Platform.
Request a free trial for GlobalVision Barcode Inspection Solution
Learn how GlobalVision has helped top Pharmaceutical Companies in quality control.
Learn more about Barcode Types and Printing
Esko Software Platform Makes Packaging Simplified
Date: April, 2016 | Category: Company | Author: Reuben Malz
Ghent (Belgium), March 16, 2016 – Esko has wholly updated its software solutions and relaunched them under the umbrella brand name “Esko Software Platform”. It’s the next version of the well-known portfolio of integrated software solutions for design, prepress, workflow automation, color management, and supply chain collaboration for packaging, labels, displays, and signs.
The Esko Software Platform is enhanced with new capabilities and delivers a unique user experience. The full range of innovations and integrations of the Esko Software Platform will be demonstrated for the first time at the upcoming Drupa 2016, scheduled for May 31 through June 10 in Düsseldorf.
Highlights of the Drupa release of the Esko Software Platform include:
A Platform for Quality Control
GlobalVision quality control solution tools are embedded in Automation Engine 16. Features now available from the automated workflow include a spell checker and automatic checking of barcodes and Braille against an approved profile. The quality check runs as a background process and results in an annotated and viewable design file for Automation Engine, WebCenter and in ArtPro+. This enables fast, systematic revision of all detected errors.
Learn More about Esko Software and GlobalVision
For more information on what’s new within Esko, please visit the Esko Software Platform.
If you want to learn more about GlobalVision’s market-leading proofreading software, request a free trial today.
________________________________________________
Learn More about How to Ensures Data Integrity
Related articles:
_________________________________________________
What does GlobalVision do?
GlobalVision’s mission is to build software that standardizes quality approvals for product packaging and critical content. Our goal is to eliminate errors and automate inspections so companies can release products with confidence. Helping Businesses Bring Confidence to Quality Control. Learn more about GlobalVision.
What is GlobalVision Quality Control?
Automate Quality Inspections for Fast and Consistent Results. Speed up the proofreading process while improving the accuracy of your work by running digital checks with GlobalVision’s automated quality control tools. Learn more about Automated Quality Control.